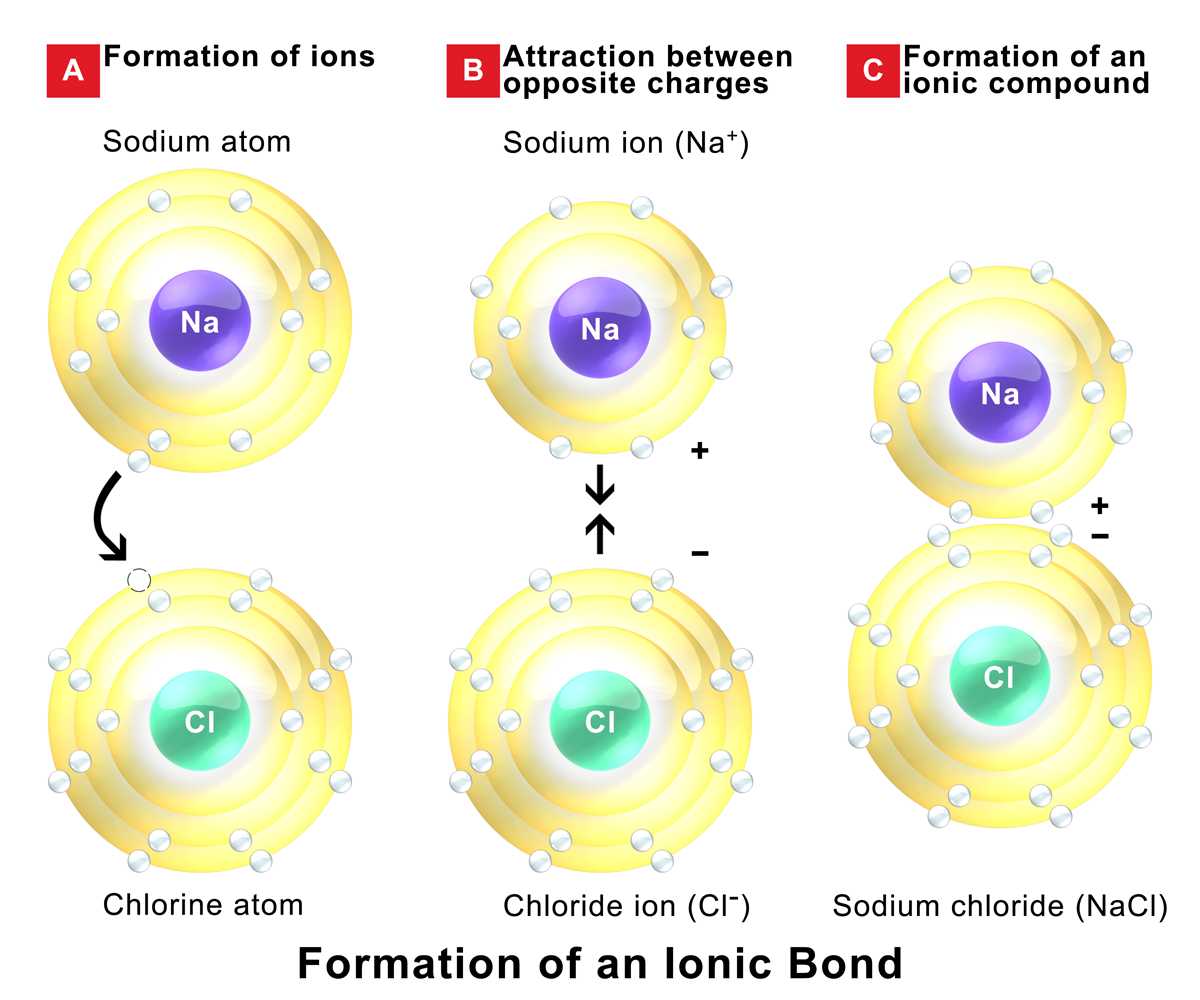
What Types Of Elements Do Ionic Bonds Form Between
What Types Of Elements Do Ionic Bonds Form Between - An atom of sodium will lose an electron and form a positive ion. It will act as a nonmetal with anegative one charge. Covalent bonding involves the sharing of electrons. An atom of chlorine will gain. Web all transition metals and rare earth metals act as positives in ionic bonding. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. This exchange results in a more stable, noble gas. Sap‑3 (eu) , sap‑3.a (lo) google classroom about transcript atoms interact with each other through the formation of chemical bonds. Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between two atoms so that ions are formed. Web how elements interact with one another depends on how their electrons are arranged and how many openings for electrons exist at the outermost region where electrons are.
The difference in electronegativity can be used to predict the type of. Web answer (1 of 3): Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Let’s consider both types of. Web all transition metals and rare earth metals act as positives in ionic bonding. Charged chemical species form when neutral atoms, or groups of atoms, lose. It will act as a nonmetal with anegative one charge. Covalent bonding involves the sharing of electrons. Web there are many types of models for ionic bonding, with the simplest being a pair potential consisting of an attractive term (between charged particles) and a repulsive term (due to. There are primarily two forms of bonding that an atom can participate in:
An atom of chlorine will gain. The difference in electronegativity can be used to predict the type of. Web there are many types of models for ionic bonding, with the simplest being a pair potential consisting of an attractive term (between charged particles) and a repulsive term (due to. Web all transition metals and rare earth metals act as positives in ionic bonding. You can also go by electronegativity. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. Web answer (1 of 3): Web typically, a metal and a nonmetal will form an ionic bond. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Which elements most often form ionic bonds?
Examples of Ionic Bonds and Ionic Compounds
Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Charged chemical species form when neutral atoms, or groups of atoms, lose. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. There is a large electronegativity. Let’s consider both.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. The difference in electronegativity can be used to predict the type of. Which elements most often form ionic bonds? There is a large electronegativity. Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds,.
Ionic Bond Definition, Types, Properties & Examples
Hydrogen can be involved in ionic bonding. Let’s consider both types of. You can also go by electronegativity. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by.
10 Notable Differences Between Ionic And Covalent Bonds Current
Hydrogen can be involved in ionic bonding. Charged chemical species form when neutral atoms, or groups of atoms, lose. Covalent bonding involves the sharing of electrons. Let’s consider both types of. Web typically, a metal and a nonmetal will form an ionic bond.
Is SiO2 Ionic or Covalent? Techiescientist
Sap‑3 (eu) , sap‑3.a (lo) google classroom about transcript atoms interact with each other through the formation of chemical bonds. Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between two atoms so that ions are formed. An atom of sodium will lose an electron and form a positive ion. Web.
Examples of Ionic Bonding YouTube
Charged chemical species form when neutral atoms, or groups of atoms, lose. An atom of chlorine will gain. This exchange results in a more stable, noble gas. Web ionic and covalent bonding. There are primarily two forms of bonding that an atom can participate in:
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Web typically, a metal and a nonmetal will form an ionic bond. An atom of sodium will lose an electron and form a positive ion. Web answer (1 of 3): Web ionic and covalent bonding.
Ionic Properties
It will act as a nonmetal with anegative one charge. An atom of sodium will lose an electron and form a positive ion. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. There is a large electronegativity. An atom of chlorine will gain.
Ionic Bond Definition, Types, Properties & Examples
Covalent bonding involves the sharing of electrons. It will act as a nonmetal with anegative one charge. Hydrogen can be involved in ionic bonding. Web ionic and covalent bonding. Which elements most often form ionic bonds?
Ionic Bonds Form When A Nonmetal And A Metal Exchange Electrons, While Covalent Bonds Form When Electrons Are Shared Between Two Nonmetals.
Web in covalent bonds, two atoms share pairs of electrons, while in ionic bonds, electrons are fully transferred between two atoms so that ions are formed. Web there are many types of models for ionic bonding, with the simplest being a pair potential consisting of an attractive term (between charged particles) and a repulsive term (due to. Which elements most often form ionic bonds? The difference in electronegativity can be used to predict the type of.
Web An Ionic Bond Is A Bond Between Two Oppositively Charged Chemical Species, A Cation And An Anion.
Covalent bonding involves the sharing of electrons. This exchange results in a more stable, noble gas. There is a large electronegativity. There are primarily two forms of bonding that an atom can participate in:
You Can Also Go By Electronegativity.
Web ionic and covalent bonding. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. An atom of chlorine will gain. It will act as a nonmetal with anegative one charge.
Web Answer (1 Of 3):
Sap‑3 (eu) , sap‑3.a (lo) google classroom about transcript atoms interact with each other through the formation of chemical bonds. Web all transition metals and rare earth metals act as positives in ionic bonding. An atom of sodium will lose an electron and form a positive ion. Let’s consider both types of.
/ionic-bond-58fd4ea73df78ca1590682ad.jpg)