In General What Determines Whether Atoms Will Form Chemical Bonds
In General What Determines Whether Atoms Will Form Chemical Bonds - Consider as an example an atom of sodium,. The first way gives rise to what is called an ionic bond. Web in general what determines whether atoms will form chemical bonds? Web they therefore, would bond together to minimize their potential energy and make them more stable. The more strongly an atom attracts the electrons in its bonds, the larger its. Web what determines whether atoms will form chemical bonds? The number of electrons in its outermost. Web there are three basic ways that the outer electrons of atoms can form bonds: Electron gain or loss can give an atom a filled outermost. Web in a mineral, the atoms are held together by chemical bonds, which derive from the electrons.
Web what determines whether an atom will form a chemical bond with another atom? Web what determines whether atoms will form chemical bonds? Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. The stability of the atom's nucleus. Some of the attractive forces are. Consider as an example an atom of sodium,. Web there are three basic ways that the outer electrons of atoms can form bonds: The number of protons in the atom's nucleus. Web in general what determines whether atoms will form chemical bonds?
Some atoms become more stable by gaining or losing an entire electron (or several electrons). Nature favors arrangements in which potential energy minimized by bonding with each other atoms decrease in. The stability of the atom's nucleus. Web the number and arrangement of electrons of an atom determine the kinds of chemical bonds that it forms and how it reacts with other atoms to form molecules. Covalent, covalent network, ionic, metallic 2. The electron arrangement of the outer energy level of an atom determines whether or not it will form. Some of the attractive forces are. Web there are three basic ways that the outer electrons of atoms can form bonds: Web in general what determines whether atoms will form chemical bonds? Web what determines whether an atom will form a chemical bond with another atom?
Knowledge Chemical Bonds
The stability of the atom's nucleus. Web the number and arrangement of electrons of an atom determine the kinds of chemical bonds that it forms and how it reacts with other atoms to form molecules. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms..
Groundbreaking movie reveals how atoms form chemical compounds
Web what determines whether atoms will form chemical bonds? To determine how many electrons are involved in the chemical bonding of two given. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. Web there are three basic ways that the outer electrons of atoms.
Why Do Atoms Form Chemical Bond ,By chemistry with concept. YouTube
The stability of the atom's nucleus. Consider as an example an atom of sodium,. The number of protons in the atom's nucleus. The electron arrangement of the outer energy level of an atom determines whether or not it will form. Click card to see definition 👆 1.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
The number of protons in the atom's nucleus. The first way gives rise to what is called an ionic bond. Web what determines whether an atom will form a chemical bond with another atom? Web in general what determines whether atoms will form chemical bonds? The more strongly an atom attracts the electrons in its bonds, the larger its.
thinkbiggerdesigns Why Do Atoms Ions
Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. The bohr model of the atom. Electron gain or loss can give an atom a filled outermost. Consider as an example an atom of sodium,. Nature favors arrangements in which potential energy minimized by bonding.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web ionic bonding and covalent bonding? Electrons can be thought of as. Web what determines whether an atom will form a chemical bond with another atom? Web in general what determines whether atoms will form chemical bonds see answer advertisement akinny answer: Web in general, what determines whether atoms will form chemical bonds?
CHEMISTRY 9TH CH 4, LECTURE 1, Why do Atoms Form Chemical Bonds
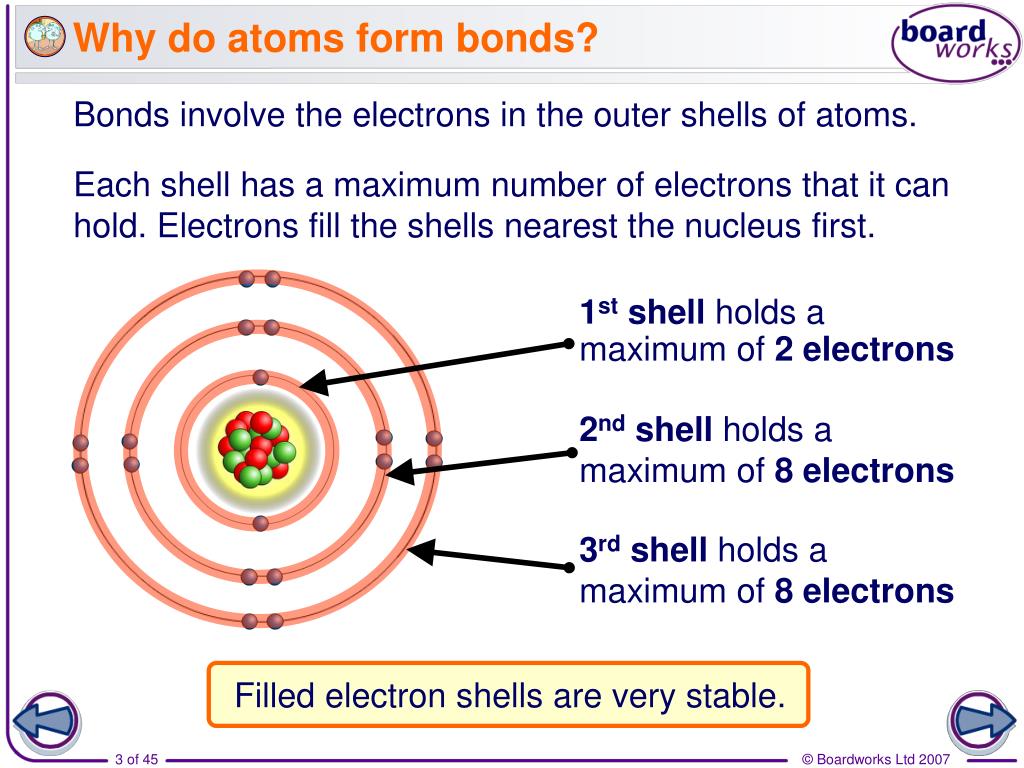
This outermost shell is known. Web there are three basic ways that the outer electrons of atoms can form bonds: The electron arrangement of electrons in the outermost energy level of the participating atoms. Web in general what determines whether atoms will form chemical bonds see answer advertisement akinny answer: Web the number and arrangement of electrons of an atom.
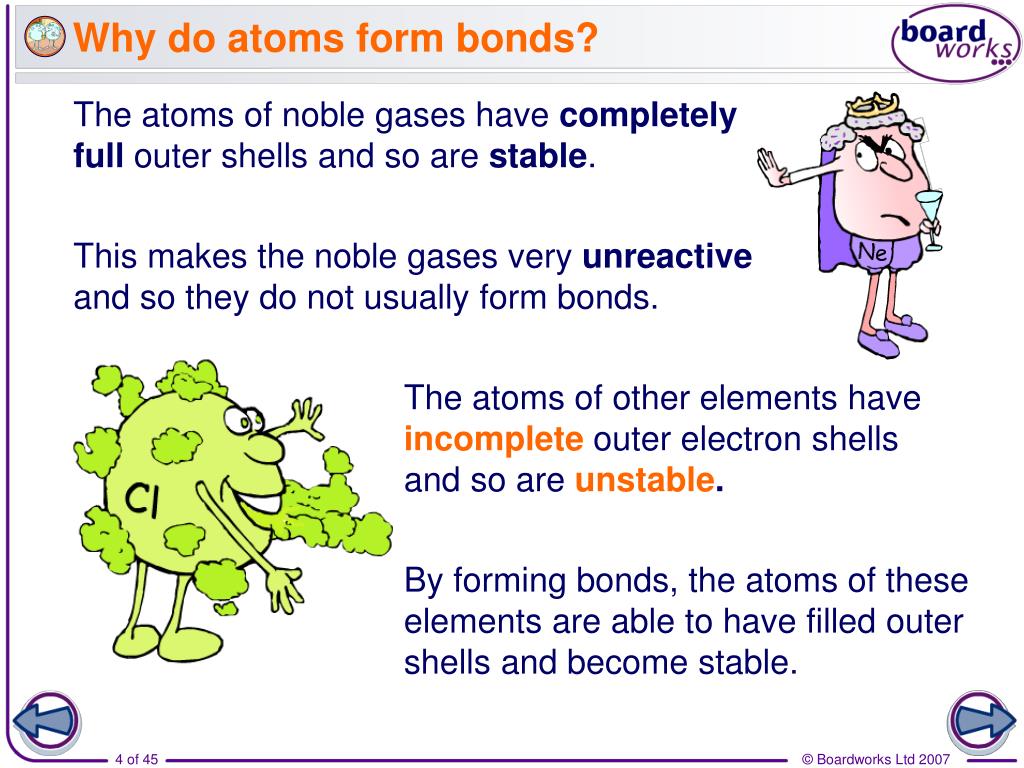
Why Do Most Atoms Form Chemical Bonds? Sciencing
Covalent, covalent network, ionic, metallic 2. Electron gain or loss can give an atom a filled outermost. An atoms first approach unit 1: The electron arrangement of the outer energy level of an atom determines whether or not it will form. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit.
A Simple Explanation of Why Atoms Form Chemical Bonds Chemical bond
The number of protons in the atom's nucleus. The stability of the atom's nucleus. Web what determines whether atoms will form chemical bonds? Web in general, what determines whether atoms will form chemical bonds? Electrons can be thought of as.
Why do atoms form bonds? YouTube
The electron arrangement of electrons in the outermost energy level of the participating atoms. Web they therefore, would bond together to minimize their potential energy and make them more stable. Web the number and arrangement of electrons of an atom determine the kinds of chemical bonds that it forms and how it reacts with other atoms to form molecules. The.
Attractive Forces Between Atoms That Are Strong Enough To Make The Linked Elements Function As A Single Unit.
Web what determines whether an atom will form a chemical bond with another atom? Web the number and arrangement of electrons of an atom determine the kinds of chemical bonds that it forms and how it reacts with other atoms to form molecules. The first way gives rise to what is called an ionic bond. The more strongly an atom attracts the electrons in its bonds, the larger its.
Some Atoms Become More Stable By Gaining Or Losing An Entire Electron (Or Several Electrons).
Web key takeaway conceptual problems contributors howard university general chemistry: Electrons can be thought of as. An atoms first approach unit 1: Consider as an example an atom of sodium,.
When They Do So, Atoms Form Ions, Or Charged Particles.
The electron arrangement of electrons in the outermost energy level of the participating atoms. Electron gain or loss can give an atom a filled outermost. The bohr model of the atom. Nature favors arrangements in which potential energy minimized by bonding with each other atoms decrease in.
Some Of The Attractive Forces Are.
Web ionic bonding and covalent bonding? Web there are three basic ways that the outer electrons of atoms can form bonds: Covalent, covalent network, ionic, metallic 2. Web what determines whether atoms will form chemical bonds?