How To Tell If A Precipitate Will Form
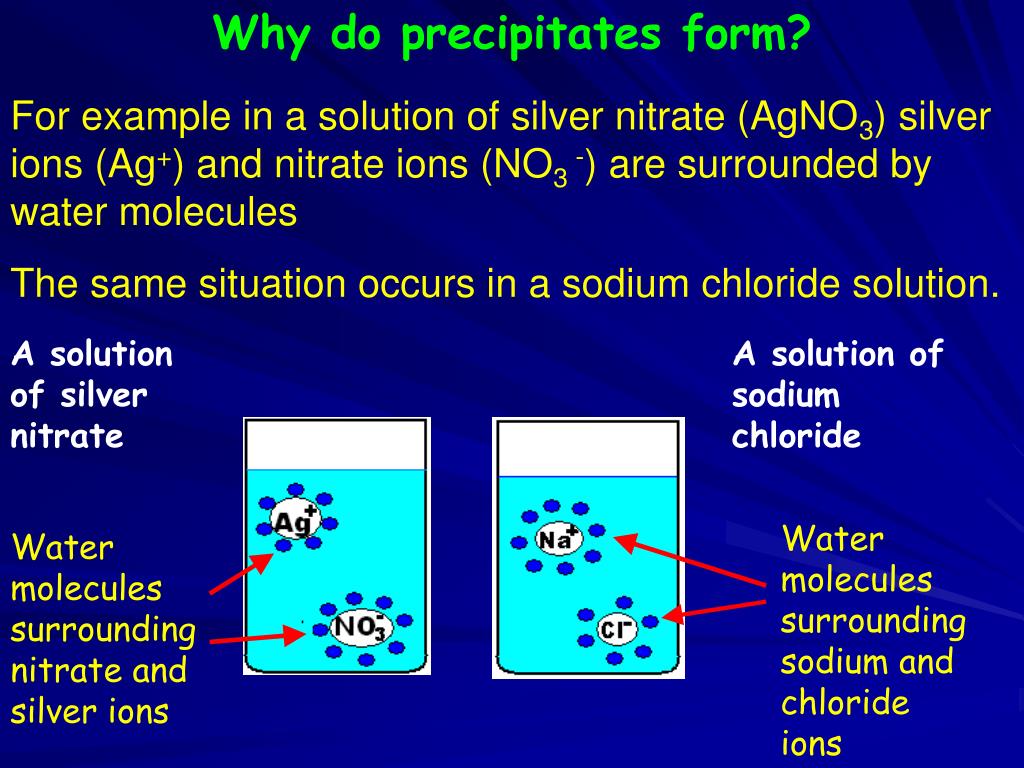
How To Tell If A Precipitate Will Form - Web for example, silver nitrate reacts with sodium chloride, yeilding silver chloride and sodium nitrate. This can occur when solutions containing. Web in this video you will learn how to predict the products of a precipitation reaction by using solubility tables. Web i know that i have to find $q_\mathrm{sp}$ and compare this to the $k_\mathrm{sp}$ given, and if $q_\mathrm{sp}$ is bigger, then a precipitate will form. 0] precipitation reactions occur when cations and anions. Web 0:00 / 5:15 example: Likewise, when substituted into the equilibrium. Web if the value of the ion product is greater than the value of the k sp, then a precipitate will form. Determining whether a precipitate will form (solubility equilibrium #3) siebert science 38.2k subscribers subscribe 539 66k views 6 years ago. Agn o3(aq) + n acl(aq) → agcl(s) +n an o3(aq) the precipitate is.
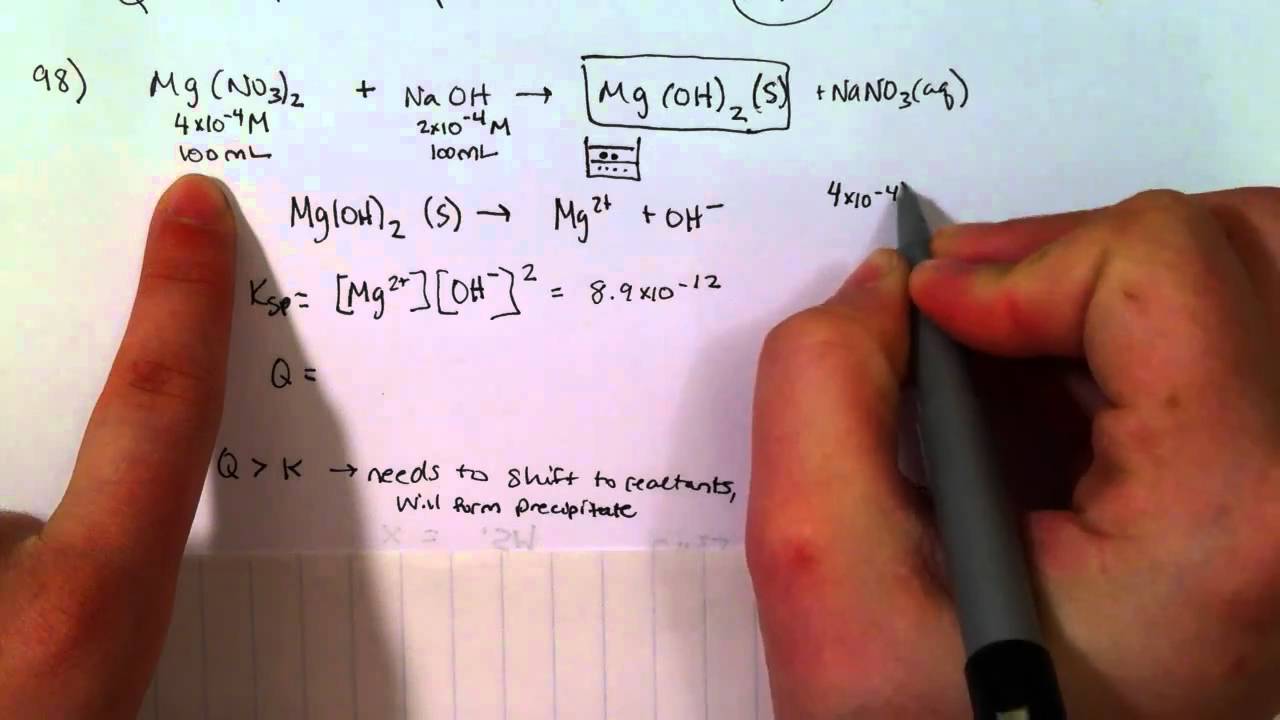
Agn o3(aq) + n acl(aq) → agcl(s) +n an o3(aq) the precipitate is. Calculation of (qc) the quotient constant and predict wether the compound forms a precipitate. Web if a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and. Ammonium (nh 4+) compounds are soluble. Web in this video you will learn how to predict the products of a precipitation reaction by using solubility tables. Web if the concentration of solute is greater than the maximum possible concentration, a precipitate will form. Web how do you know if a precipitate will form? Web a precipitate forms if the product of the reaction of the ions is insoluble in water. Web 0:00 / 5:15 example: Likewise, when substituted into the equilibrium.
Silver nitrate and potassium chloride is a. Web i know that i have to find $q_\mathrm{sp}$ and compare this to the $k_\mathrm{sp}$ given, and if $q_\mathrm{sp}$ is bigger, then a precipitate will form. Precipitation reactions combine aqueous reactants. Web a precipitate forms if the product of the reaction of the ions is insoluble in water. Web if the value of the ion product is greater than the value of the k sp, then a precipitate will form. Web goes over an example problem about predicting if a precipitate will form or not. What is precipitation give example? Likewise, when substituted into the equilibrium. The formation of the precipitate lowers the concentration of each of the ions until. On:july 7, 2022 asked by:
How To Determine Solubility sharedoc
Web i know that i have to find $q_\mathrm{sp}$ and compare this to the $k_\mathrm{sp}$ given, and if $q_\mathrm{sp}$ is bigger, then a precipitate will form. Agn o3(aq) + n acl(aq) → agcl(s) +n an o3(aq) the precipitate is. Web how do you know if a precipitate will form? Web 0:00 / 5:15 example: Web if a precipitate is formed.
Predicting formation of a precipitate YouTube
0] precipitation reactions occur when cations and anions. Silver nitrate and potassium chloride is a. Web 🎯 want to ace chemistry? A precipitate is a solid formed in a chemical reaction that is different from either of the reactants. Web a precipitate forms if the product of the reaction of the ions is insoluble in water.
SOLVEDCalculate whether a precipitate will form
Precipitation reactions combine aqueous reactants. Web 0:00 / 5:15 example: Web one is the formation of a precipitate. This can occur when solutions containing. On:july 7, 2022 asked by:
Precipitate Definition and Example in Chemistry
Agn o3(aq) + n acl(aq) → agcl(s) +n an o3(aq) the precipitate is. Calculation of (qc) the quotient constant and predict wether the compound forms a precipitate. Web one is the formation of a precipitate. Precipitation reactions combine aqueous reactants. Web if a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water.
Solved Will precipitate form from the combination of 50.0 mL
Likewise, when substituted into the equilibrium. Ammonium (nh 4+) compounds are soluble. Precipitation reactions combine aqueous reactants. The formation of the precipitate lowers the concentration of each of the ions until. Web a precipitate forms if the product of the reaction of the ions is insoluble in water.
Q Will a Precipitate Form (Z.15.97) YouTube
Alkali metal (group ia) compounds are soluble. Ammonium (nh 4+) compounds are soluble. Agn o3(aq) + n acl(aq) → agcl(s) +n an o3(aq) the precipitate is. Web one is the formation of a precipitate. Web if the value of the ion product is greater than the value of the k sp, then a precipitate will form.
Solved 21. Will a precipitate form when 0.1 M aqueous
Web in this video you will learn how to predict the products of a precipitation reaction by using solubility tables. Web a precipitate forms if the product of the reaction of the ions is insoluble in water. If the product of the concentrations of ions after mixing is greater than the ksp value, then a. 135 views 1 year ago.
PPT Why do precipitates form? PowerPoint Presentation, free download
Web in this video you will learn how to predict the products of a precipitation reaction by using solubility tables. Web for example, silver nitrate reacts with sodium chloride, yeilding silver chloride and sodium nitrate. Alkali metal (group ia) compounds are soluble. This can occur when solutions containing. Web i know that i have to find $q_\mathrm{sp}$ and compare this.
Complete the reaction and determine if a precipitate forms 4 YouTube
Web 🎯 want to ace chemistry? Web for example, silver nitrate reacts with sodium chloride, yeilding silver chloride and sodium nitrate. This can occur when solutions containing. Likewise, when substituted into the equilibrium. Alkali metal (group ia) compounds are soluble.
Question Video Determining Which Reaction Will Produce a Precipitate
Likewise, when substituted into the equilibrium. Silver nitrate and potassium chloride is a. Web if the concentration of solute is greater than the maximum possible concentration, a precipitate will form. Calculation of (qc) the quotient constant and predict wether the compound forms a precipitate. What is precipitation give example?
This Can Occur When Solutions Containing.
Web 🎯 want to ace chemistry? Web i know that i have to find $q_\mathrm{sp}$ and compare this to the $k_\mathrm{sp}$ given, and if $q_\mathrm{sp}$ is bigger, then a precipitate will form. Agn o3(aq) + n acl(aq) → agcl(s) +n an o3(aq) the precipitate is. Likewise, when substituted into the equilibrium.
Web In This Video You Will Learn How To Predict The Products Of A Precipitation Reaction By Using Solubility Tables.
Web 0:00 / 5:15 example: The formation of the precipitate lowers the concentration of each of the ions until. Calculation of (qc) the quotient constant and predict wether the compound forms a precipitate. Web for example, silver nitrate reacts with sodium chloride, yeilding silver chloride and sodium nitrate.
Silver Nitrate And Potassium Chloride Is A.
Web if a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and. Determining whether a precipitate will form (solubility equilibrium #3) siebert science 38.2k subscribers subscribe 539 66k views 6 years ago. On:july 7, 2022 asked by: Ammonium (nh 4+) compounds are soluble.
Web A Precipitate Forms If The Product Of The Reaction Of The Ions Is Insoluble In Water.
Web one is the formation of a precipitate. A precipitate is a solid formed in a chemical reaction that is different from either of the reactants. Web goes over an example problem about predicting if a precipitate will form or not. Web if the concentration of solute is greater than the maximum possible concentration, a precipitate will form.



:max_bytes(150000):strip_icc()/precipitate-589cb8953df78c47581a9014.jpg)