How Many Bonds Can Bromine Form
How Many Bonds Can Bromine Form - Web bromine is capable of forming one bond when it is in its elemental form. Web expert answer 100% (6 ratings) carbon atom has four valence electr. However, it can also form multiple bonds with other elements. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet For example, it can form two bonds with. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web how many bonds can bromine make? Web how many bonds will bromine most likely form?4 , 2, 1, or 7. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group.
Oxygen will normally have tow bonds, although it may have three in certain molecules (ozone is an example). Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet Two single bonds and a double bond. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next The maximum number of bonds. For example, it can form two bonds with. Modeling lonic and covalent bonds part 1: Web how many bonds can bromine make? Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Web bromine will normally form one covalent bond.
Web bromine compounds are compounds containing the element bromine (br). 6 which elements tend to form covalent bonds? Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. Web bromine will normally form one covalent bond. Let's illustrate how a covalent bond forms between. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Web bromine will normally form one covalent bond. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Group 5a (15) elements such as nitrogen have five valence electrons in. However, it can also form multiple bonds with other elements.
How Many Bonds Can Nitrogen Form Jacks Of Science
Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet However, it can also form multiple bonds with other elements. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. Bromine, which belongs to.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web a chemical bond that forms between nonmetals and/or metalloids that is the result of sharing their valence electrons. View the full answer transcribed image text: 6 which elements tend to form covalent bonds? However, it can also form multiple bonds with other elements. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is.
Solved how many bonds can/should be drawn to the following
1 bond how many bonds can sulfur form with neighboring atoms in a compound? View the full answer transcribed image text: Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Let's illustrate how a covalent bond forms between. Web.
How Many Bonds Can Nitrogen Form Jacks Of Science
Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence The maximum number of bonds. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Web bromine will typically form one.
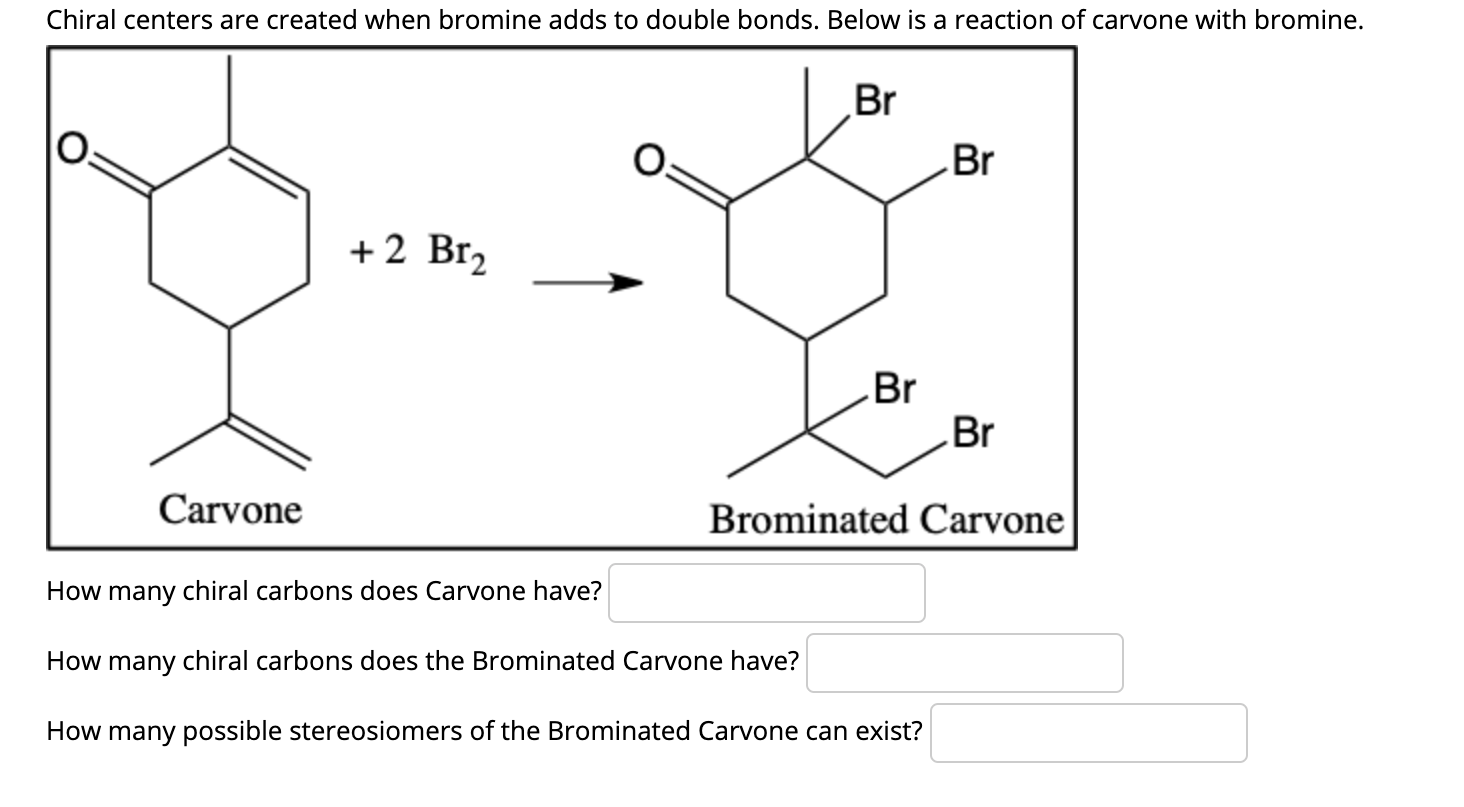
Solved Chiral centers are created when bromine adds to
Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Web bromine compounds are compounds containing the element bromine (br). A.
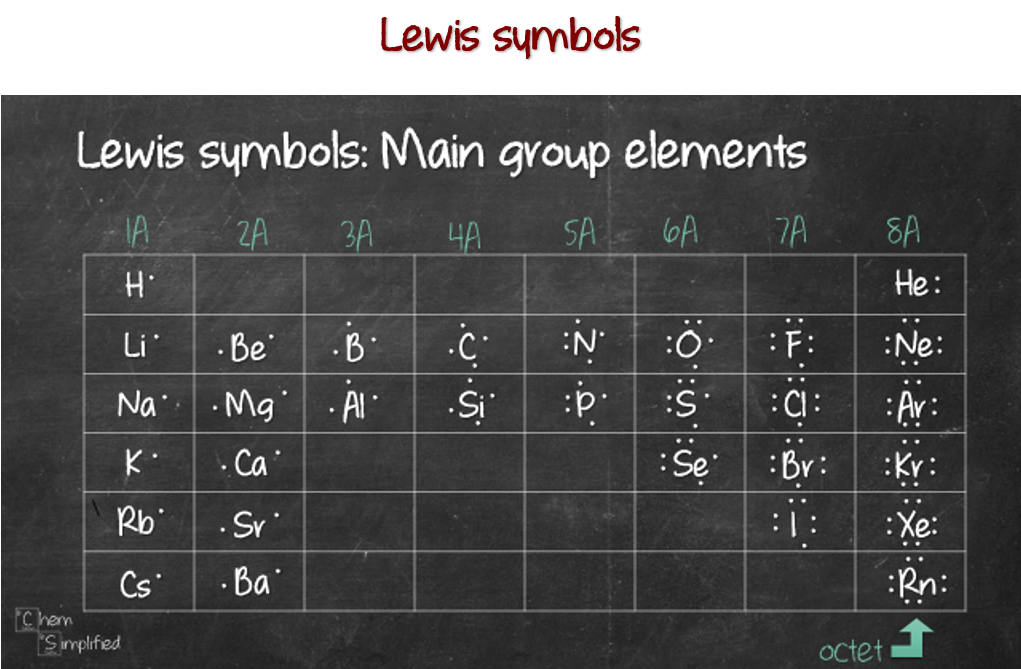
How to Predict number of bonds each element forms ChemSimplified
Two single bonds and a double bond. For example, it can form two bonds with. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. 1 bond how many bonds can sulfur form with neighboring atoms in a compound? Web bromine will typically form one bond, as it is a halogen.
Physical and Chemical Properties Bromine
View the full answer transcribed image text: The maximum number of bonds. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more.
How Can We Find A Electron Configuration For Bromine (Br)
Web how many bonds will bromine most likely form?4 , 2, 1, or 7. Which of the following situations meet the bonding requirement for carbon atoms. Web expert answer 100% (6 ratings) carbon atom has four valence electr. For example, it can form two bonds with. Web bromine will normally form one covalent bond.
According to the following reaction, how... Chemistry
Web expert answer 100% (6 ratings) carbon atom has four valence electr. A single bond and two double bonds. For example, it can form two bonds with. 1 pm = 1 × 10 −12 m). However, it can also form multiple bonds with other elements.
HONC 1234 ChemSimplified
For example, it can form two bonds with. Web bromine will typically form one bond, as it is a halogen. Group 5a (15) elements such as nitrogen have five valence electrons in. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet Modeling.
Web Expert Answer 100% (6 Ratings) Carbon Atom Has Four Valence Electr.
Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Web bromine will normally form one covalent bond. Web how many bonds can bromine make? The maximum number of bonds.
Web The Bond In A Hydrogen Molecule, Measured As The Distance Between The Two Nuclei, Is About 7.4 × 10 −11 M, Or 74 Picometers (Pm;
1 pm = 1 × 10 −12 m). Web bromine will typically form one bond, as it is a halogen. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1.
Web If Either Iodine Or Bromine Were To Given Up Valence Electrons To Form A Cation, They Would Have To Give Up All Seven Valence Electrons To Reveal The Next
Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. Web bromine will normally form one covalent bond. However, it can also form multiple bonds with other elements. Let's illustrate how a covalent bond forms between.
The Valence Electrons In Al Is Three.
Group 5a (15) elements such as nitrogen have five valence electrons in. Two single bonds and a double bond. For example, it can form two bonds with. Web how many bonds will bromine most likely form?4 , 2, 1, or 7.