Why Do Metals Form Cations
Why Do Metals Form Cations - Web metallic atoms hold some of their electrons relatively loosely. Metals are good conductors because they have free electrons. Web acetylcholine is a cation that is a neurotransmitter, responsible for the transference of nerve impulses. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the. Web atoms lose electrons from their outer shell when they form positive ions, called cations. Energy is released when electrons are removed from metal ions. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. This is actually one of the chemical properties. These ions are positive because they contain more protons than electrons. The more vigorous its reactions are;
Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the. Alkali and alkaline earth metals tend to form cations because when losing 1 or. Web metallic atoms hold some of their electrons relatively loosely. Web 1 language a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. These ions are positive because they contain more protons than electrons. We will take a closer look at why is that so. The solvation number, n, determined by a variety of. This is actually one of the chemical properties. Web atoms lose electrons from their outer shell when they form positive ions, called cations. Energy is released when electrons are removed from metal ions.
This is the typical behavior. Alkali and alkaline earth metals tend to form cations because when losing 1 or. Web it is generally known that metals tend to form cations. Consequently, they tend to lose electrons and form cations. Metals are good conductors because they have free electrons. Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to react with simple lewis bases to form metal complexes. This is actually one of the chemical properties. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web atoms lose electrons from their outer shell when they form positive ions, called cations. We will take a closer look at why is that so.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Most other nonmetals typically form anions (e.g. Silver and copper are the two best conductors of heat and electricity. Metals are good conductors because they have free electrons. Alkali and alkaline earth metals tend to form cations because when losing 1 or. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions.
How To Find Electron Charge On Periodic Table Food Ideas
The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. The solvation number, n, determined by a variety of. These ions are positive because they contain more protons than electrons. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that.
Solved Question 6 (1 point) Why do metals tend to form
Potassium cations are common inside animal cells, and. Web it is generally known that metals tend to form cations. Web the alkali metals tend to form +1 cations. Web alkali metals and alkaline earth metals always form cations. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few.
10 28 How Many Electrons Do Atoms Gain Lose
It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web atoms lose electrons from their outer shell when they form positive ions, called cations. Web metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Potassium cations are common inside animal cells,.
PPT Naming Ionic Compounds PowerPoint Presentation, free download
The solvation number, n, determined by a variety of. Potassium cations are common inside animal cells, and. We will take a closer look at why is that so. Web the alkali metals tend to form +1 cations. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal.
is incorreer? all s area eleseis erand e are metals b, all p area
Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the. Web atoms lose electrons from their outer shell when they form positive ions, called cations. This is actually one of the chemical properties. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises.
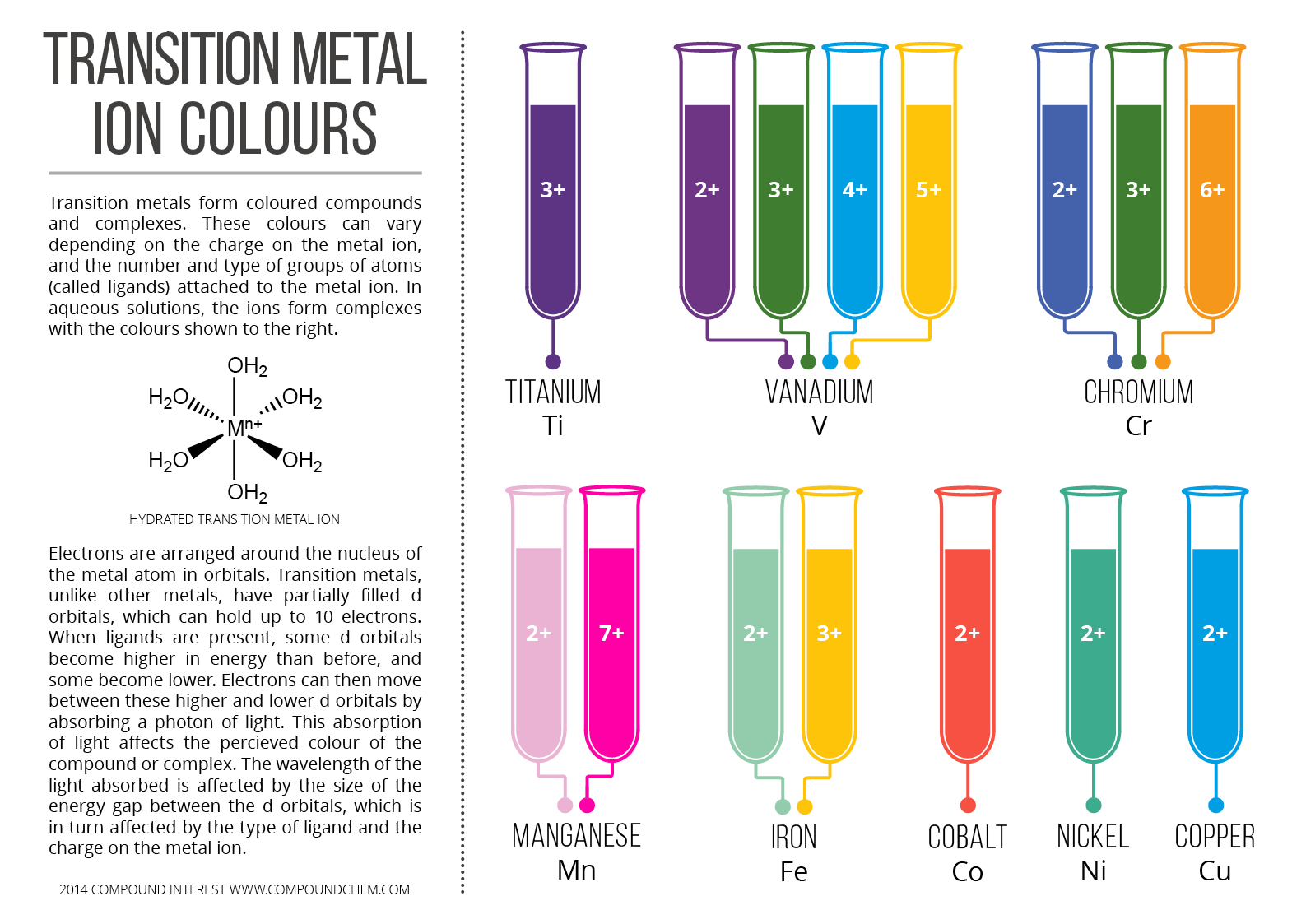
Colours of Transition Metal Ions in Aqueous Solution [Infographic
Alkali and alkaline earth metals tend to form cations because when losing 1 or. This is actually one of the chemical properties. Web 1 language a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. The solvation number, n, determined by a variety of. Most other.
Chem matters ch6_ionic_bond
This is actually one of the chemical properties. Web metal elements form positively charged ions called cations because they are located on the left side of the periodic table. The solvation number, n, determined by a variety of. Silver and copper are the two best conductors of heat and electricity. Cation formation is favored by the relatively low ionization energies.
Why do metals form cations and non metals form anions?
The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. The more vigorous its reactions are; The solvation number, n, determined by a variety of. Consequently, they tend to lose electrons and form cations. Web because of their low positive charge (+1) and relatively large ionic radii, alkali.
Do metals form anions or cations quizlet? Book Vea
Web metallic atoms hold some of their electrons relatively loosely. Consequently, they tend to lose electrons and form cations. Potassium cations are common inside animal cells, and. These ions are positive because they contain more protons than electrons. Energy is released when electrons are removed from metal ions.
Web Cations Are Atoms That Contain A Positive Charge, And They Are Formed When The Atoms Lose Electrons Which Are Negatively Charged.
This is the typical behavior. Most other nonmetals typically form anions (e.g. This is actually one of the chemical properties. The solvation number, n, determined by a variety of.
These Ions Are Positive Because They Contain More Protons Than Electrons.
Web atoms lose electrons from their outer shell when they form positive ions, called cations. Web 1 language a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m (h 2 o) n] z+. Web it is generally known that metals tend to form cations. We will take a closer look at why is that so.
Web The Alkali Metals Tend To Form +1 Cations.
Silver and copper are the two best conductors of heat and electricity. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. The more easily it loses electrons in reactions to form positive ions (cations) the table summarises some reactions of metals in the. Web alkali metals and alkaline earth metals always form cations.
Potassium Cations Are Common Inside Animal Cells, And.
Energy is released when electrons are removed from metal ions. Alkali and alkaline earth metals tend to form cations because when losing 1 or. Cation formation is favored by the relatively low ionization energies of the free metal (which makes it easier to form the. Consequently, they tend to lose electrons and form cations.