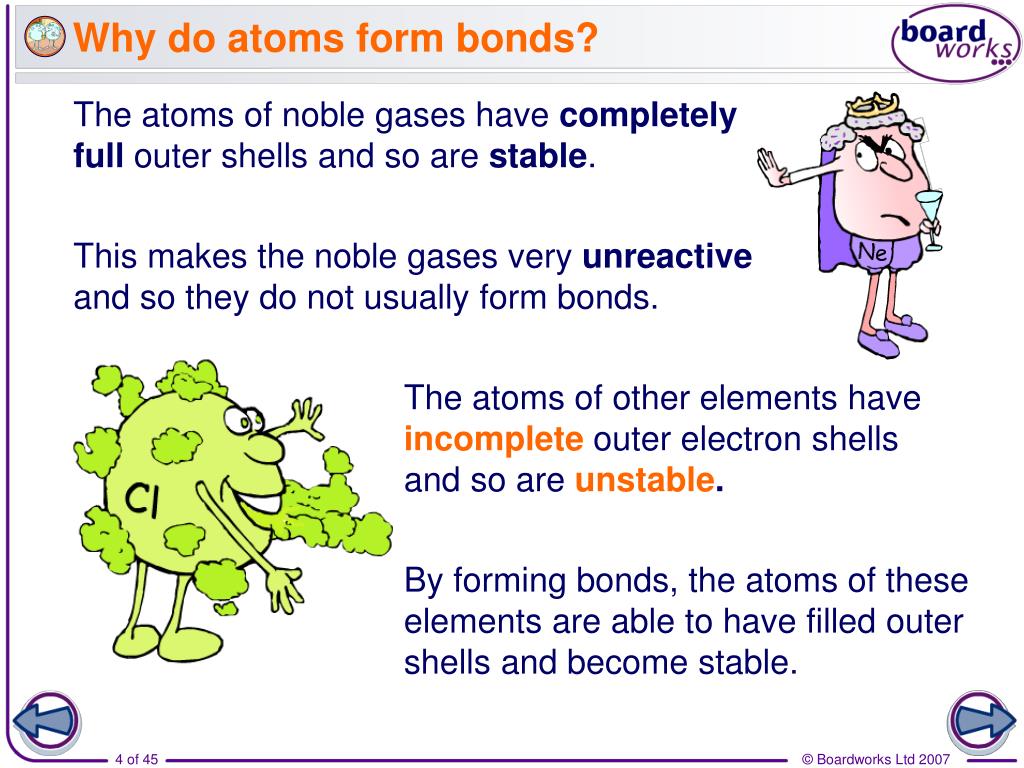
Why Do Atoms Form Bonds With Other Atoms
Why Do Atoms Form Bonds With Other Atoms - Web atoms have to be close together to form a bond. Web a double bond is formed when two atoms use two electron pairs to form two covalent bonds; Atomic structure of carbon atom showing the particles of an atom: The combination of multiple atoms, or chemical bonding, forms molecules. Web when atoms form bonds, they can achieve a stable electron arrangement. Web the atoms of molecules are linked together through a reaction known as chemical bonding. The more “crowded” a given space is with atoms, the more likely it is that. In fact, they have to collide (bump together). To achieve a stable electron arrangement atoms can lose, gain or share electrons. A triple bond results when two atoms share three electron pairs to form three.
Atomic structure of carbon atom showing the particles of an atom: Web atoms are individual units made up of protons, neutrons, and electrons. There are several types of bond. Web if you learned in chemistry that some atoms tend to gain or lose electrons or form bonds with each other, those facts remain true even when the atoms or molecules are part of a. Elements are listed on the periodic table. Web main types of chemical bonds. The more “crowded” a given space is with atoms, the more likely it is that. The bond may result from the electrostatic. Web to achieve a full valence electrons shell. Atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons.
This outermost shell is known as. There are several types of bond. An ionic bond is formed when one. The combination of multiple atoms, or chemical bonding, forms molecules. Elements are listed on the periodic table. Atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons. Web atoms are individual units made up of protons, neutrons, and electrons. Web a double bond is formed when two atoms use two electron pairs to form two covalent bonds; A pure substance made from only one type of atom is called an element. A triple bond results when two atoms share three electron pairs to form three.
why do atoms form bonds with other atoms
Web the atoms of molecules are linked together through a reaction known as chemical bonding. Web atoms can form strong bonds with each other, making molecules. Web when atoms form bonds, they can achieve a stable electron arrangement. A triple bond results when two atoms share three electron pairs to form three. Atomic structure of carbon atom showing the particles.
PPT Chemical Bonding What and Why PowerPoint Presentation, free
A pure substance made from only one type of atom is called an element. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Web if you learned in chemistry that some atoms tend to gain or lose electrons or form bonds with each other, those facts remain.
PPT Ions PowerPoint Presentation, free download ID6738771
Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Elements are listed on the periodic table. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Web when atoms form bonds, they can achieve a stable electron arrangement. An ionic bond is.
Why do atoms have electrons? How It Works Magazine
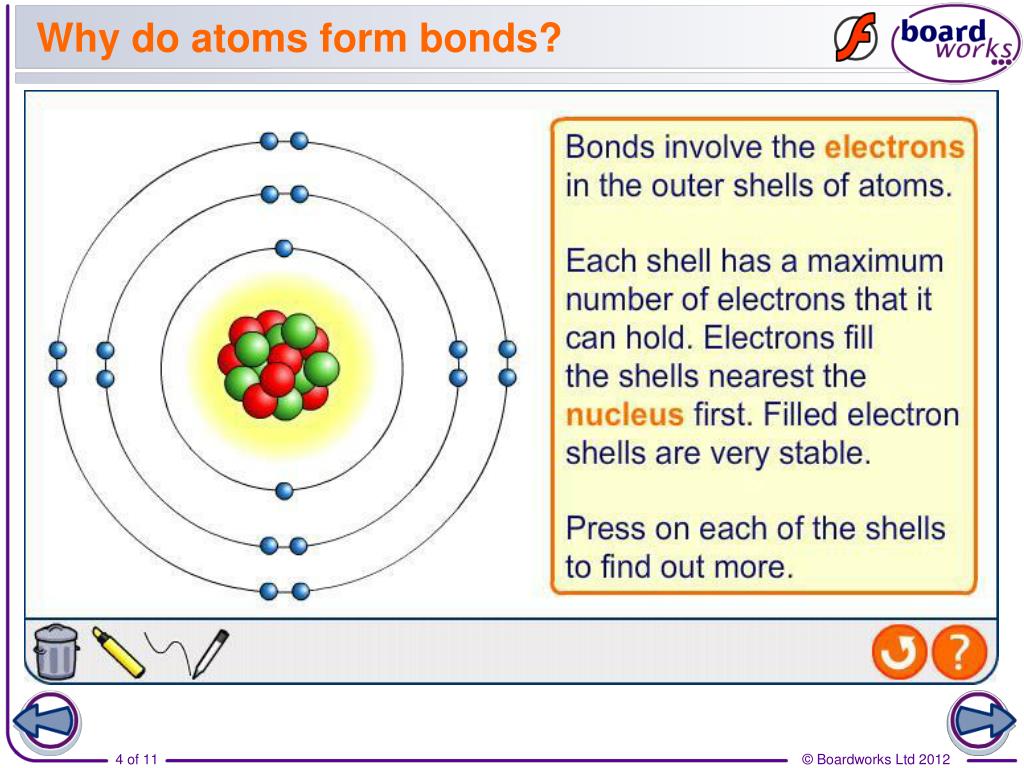
An ionic bond is formed when one. Web main types of chemical bonds. Web atoms can form strong bonds with each other, making molecules. Web atoms have to be close together to form a bond. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms.
Biochemistry Honors
The combination of multiple atoms, or chemical bonding, forms molecules. The more “crowded” a given space is with atoms, the more likely it is that. An ionic bond is formed when one. Atomic structure of carbon atom showing the particles of an atom: A pure substance made from only one type of atom is called an element.
Why do atoms form bonds? Video] O Level Secondary Chemistry
Web atoms are individual units made up of protons, neutrons, and electrons. There are several types of bond. Atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons. Web atoms have to be close together to form a bond. Elements are listed on the periodic table.
thinkbiggerdesigns Why Do Atoms Ions
There are several types of bond. Web to achieve a full valence electrons shell. Web main types of chemical bonds. Web if you learned in chemistry that some atoms tend to gain or lose electrons or form bonds with each other, those facts remain true even when the atoms or molecules are part of a. The combination of multiple atoms,.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
To achieve a stable electron arrangement atoms can lose, gain or share electrons. A triple bond results when two atoms share three electron pairs to form three. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Web atoms are individual units made up of protons, neutrons, and.
Why Do Atoms Form Chemical Bond in Urdu Hindi Lecture /Chemistry for
Web atoms can form strong bonds with each other, making molecules. Web atoms have to be close together to form a bond. The bond may result from the electrostatic. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Web main types of chemical bonds.
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609
Web the atoms of molecules are linked together through a reaction known as chemical bonding. Web atoms are individual units made up of protons, neutrons, and electrons. Web atoms can form strong bonds with each other, making molecules. Elements are listed on the periodic table. Web main types of chemical bonds.
Web Atoms Have To Be Close Together To Form A Bond.
Web the atoms of molecules are linked together through a reaction known as chemical bonding. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Web the number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. Web atoms can form strong bonds with each other, making molecules.
An Ionic Bond Is Formed When One.
There are several types of bond. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Web when atoms form bonds, they can achieve a stable electron arrangement. Atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons.
Elements Are Listed On The Periodic Table.
In fact, they have to collide (bump together). Web atoms are individual units made up of protons, neutrons, and electrons. A pure substance made from only one type of atom is called an element. Web a double bond is formed when two atoms use two electron pairs to form two covalent bonds;
The More “Crowded” A Given Space Is With Atoms, The More Likely It Is That.
Atomic structure of carbon atom showing the particles of an atom: The combination of multiple atoms, or chemical bonding, forms molecules. The bond may result from the electrostatic. A triple bond results when two atoms share three electron pairs to form three.

![Why do atoms form bonds? Video] O Level Secondary Chemistry](https://icandochemistry942105908.files.wordpress.com/2021/06/thumb-1.png?w=1200)