When Metals Form Ions They Tend To Do So By
When Metals Form Ions They Tend To Do So By - Compounds that do not contain ions, but. Losing electrons and forming negative ions3. In the chemistry of the transition elements, the 4s orbital behaves as the. Thus metals are electropositive elements. Is there a simple way to tell which elements have multiple cations, or is it pure memorization? Groups 1 and 2 are called the alkali. This is actually one of the chemical properties of metals and nonmetals: Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Web they do it because they have few electrons in their outermost answer:1. A cations b anions c cations and anions d do not form ions easy open in app solution verified by toppr correct option is a) metals lose electrons in.
This is actually one of the chemical properties of metals and nonmetals: Web metals tend to form: The charges of cations formed by the representative metals may be determined readily. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Is there a simple way to tell which elements have multiple cations, or is it pure memorization? In the chemistry of the transition elements, the 4s orbital behaves as the. Web so my question is: Web compounds that contain ions are called ionic compounds. Metal elements form positively charged ions called cations because they are located on the left side of the periodic table. Compounds that do not contain ions, but.
Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. Losing electrons and forming negative ions3. In the chemistry of the transition elements, the 4s orbital behaves as the. Web metal atoms lose electrons from their outer shell when they form ions: Ionic compounds generally form from metals and nonmetals. Metals tend to form cations, while nonmetals tend to form anions. The ions are positive, because they have more protons than electrons the ions formed have full outer. Thus metals are electropositive elements. Web metals form ions by losing electrons. This is actually one of the chemical properties of metals and nonmetals:
CH103 CHAPTER 4 Ions and Ionic Compounds Chemistry
A cations b anions c cations and anions d do not form ions easy open in app solution verified by toppr correct option is a) metals lose electrons in. Web as you may notice, they can form ions by either losing or gaining electron in 4s orbital. Compounds that do not contain ions, but instead consist of. Thus metals are.
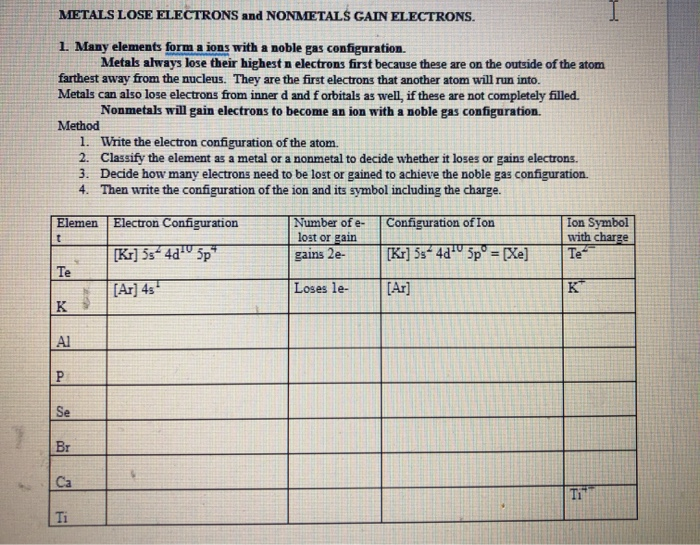
Solved METALS LOSE ELECTRONS and NONMETALS GAIN ELECTRONS.
Compounds that do not contain ions, but instead consist of. Web metals tend to form: Web compounds that contain ions are called ionic compounds. Web compounds that contain ions are called ionic compounds. Transition metals often form more than one.
Pin em Science, bitch!
Web metals form ions by losing electrons. Metals tend to form cations, while nonmetals tend to form anions. Web answer (1 of 4): When metals form ions they tend to do so by1. Groups 1 and 2 are called the alkali.
table of elements chart
Thus metals are electropositive elements. The ions are positive, because they have more protons than electrons the ions formed have full outer. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Web metals tend to form positive ions because their electron structure causes them to do so. They do it because they have few electrons in their outermost.
Student Resources CHEMISTRY BATZ
Thus metals are electropositive elements. Web they do it because they have few electrons in their outermost answer:1. This is actually one of the chemical properties of metals and nonmetals: In the chemistry of the transition elements, the 4s orbital behaves as the. Web compounds that contain ions are called ionic compounds.
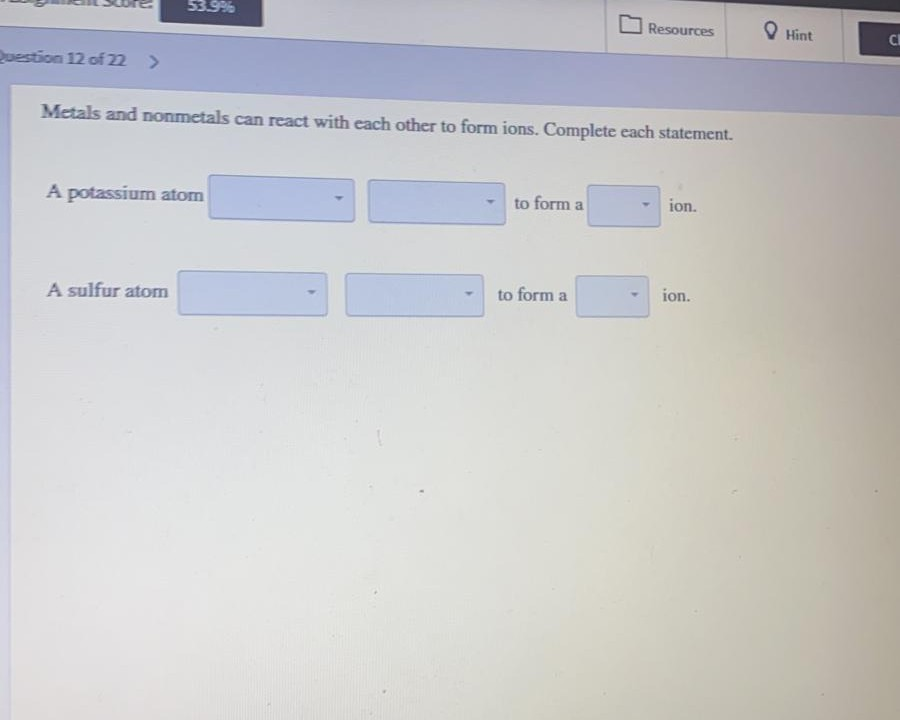
Solved 53.9 Resources Hint СІ Question 12 of 22 > Metals
Web metals form ions by losing electrons. Web metals tend to form: Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. A cations b anions c cations and anions d do not form ions easy open in app solution verified by toppr correct option is a) metals lose.
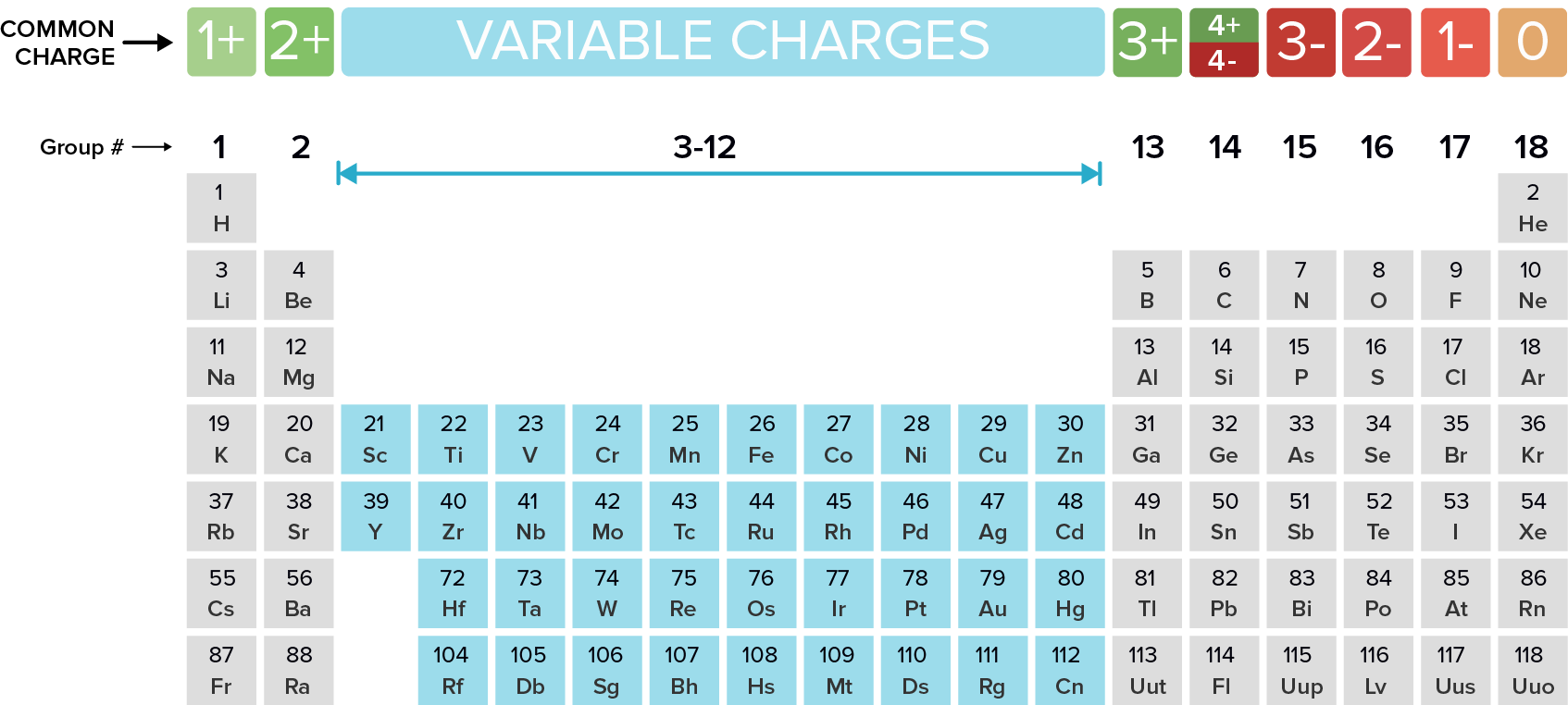
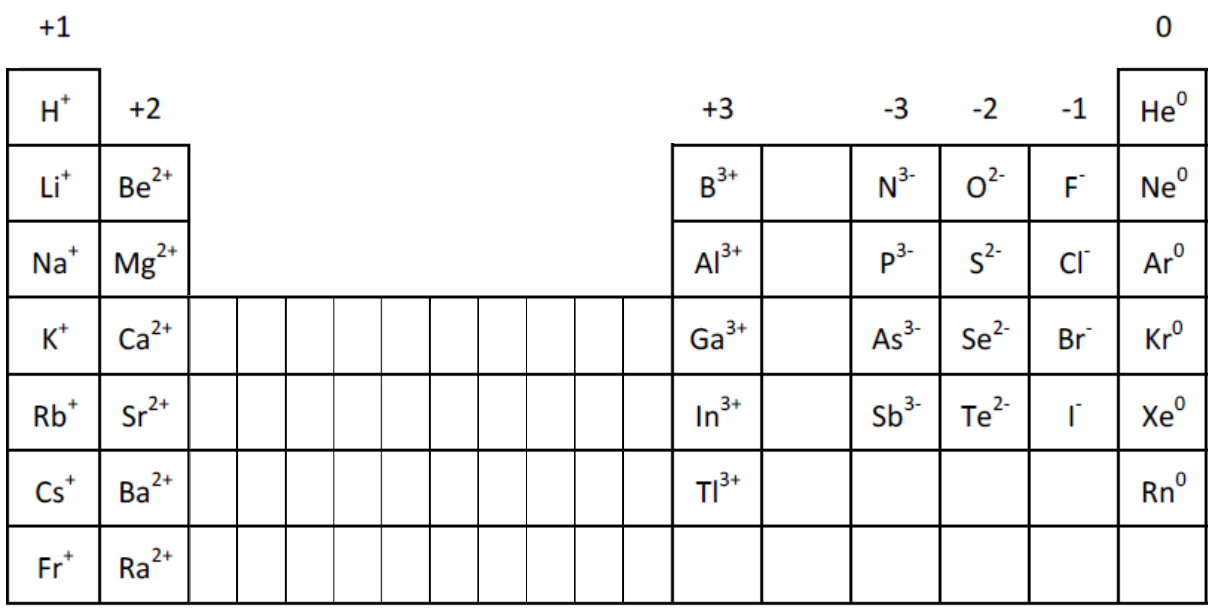
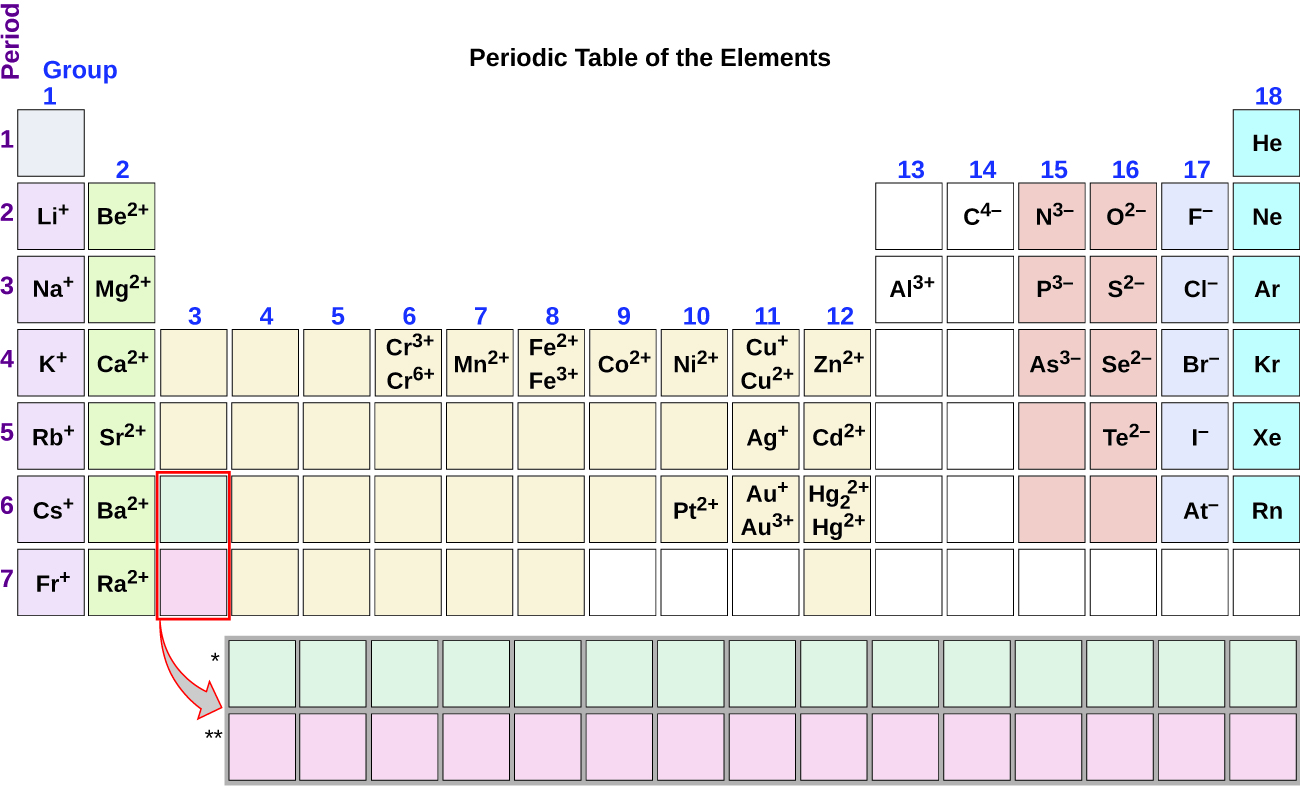
Ion Charge from Periodic Table NemoQuiz
Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the. Metal elements form.
Use data above answer questions 2,3,4 EXPERIMENT Activity of Metals
We need to talk briefly about what this means, so put on your thinking. When metals form ions they tend to do so by1. So rather than accept more electrons, it is much more. Losing electrons and forming negative ions3. Ionic compounds generally form from metals and nonmetals.
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Web compounds that contain ions are called ionic compounds. Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. The ions are positive, because they have more protons than electrons.
Colours of Transition Metal Ions in Aqueous Solution Compound Interest
In the chemistry of the transition elements, the 4s orbital behaves as the. When metals form ions they tend to do so by1. So rather than accept more electrons, it is much more. Web metals form ions by losing electrons. Web metals tend to form:
Thus Metals Are Electropositive Elements.
Web answer (1 of 4): Compounds that do not contain ions, but. Losing electrons and forming negative ions3. Web metals tend to form:
Web Metals Form Ions By Losing Electrons.
A cations b anions c cations and anions d do not form ions easy open in app solution verified by toppr correct option is a) metals lose electrons in. Transition metals often form more than one. Thus metals are electropositive elements. Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals.
They Do It Because They Have Few Electrons In Their Outermost Shells.
Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic configuration, as most metals have electrons. Web metals tend to form positive ions because their electron structure causes them to do so. So rather than accept more electrons, it is much more. When metals form ions they tend to do so by1.
In The Chemistry Of The Transition Elements, The 4S Orbital Behaves As The.
Web all elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metals tend to form cations, while nonmetals tend to form anions. Ionic compounds generally form from metals and nonmetals. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the.