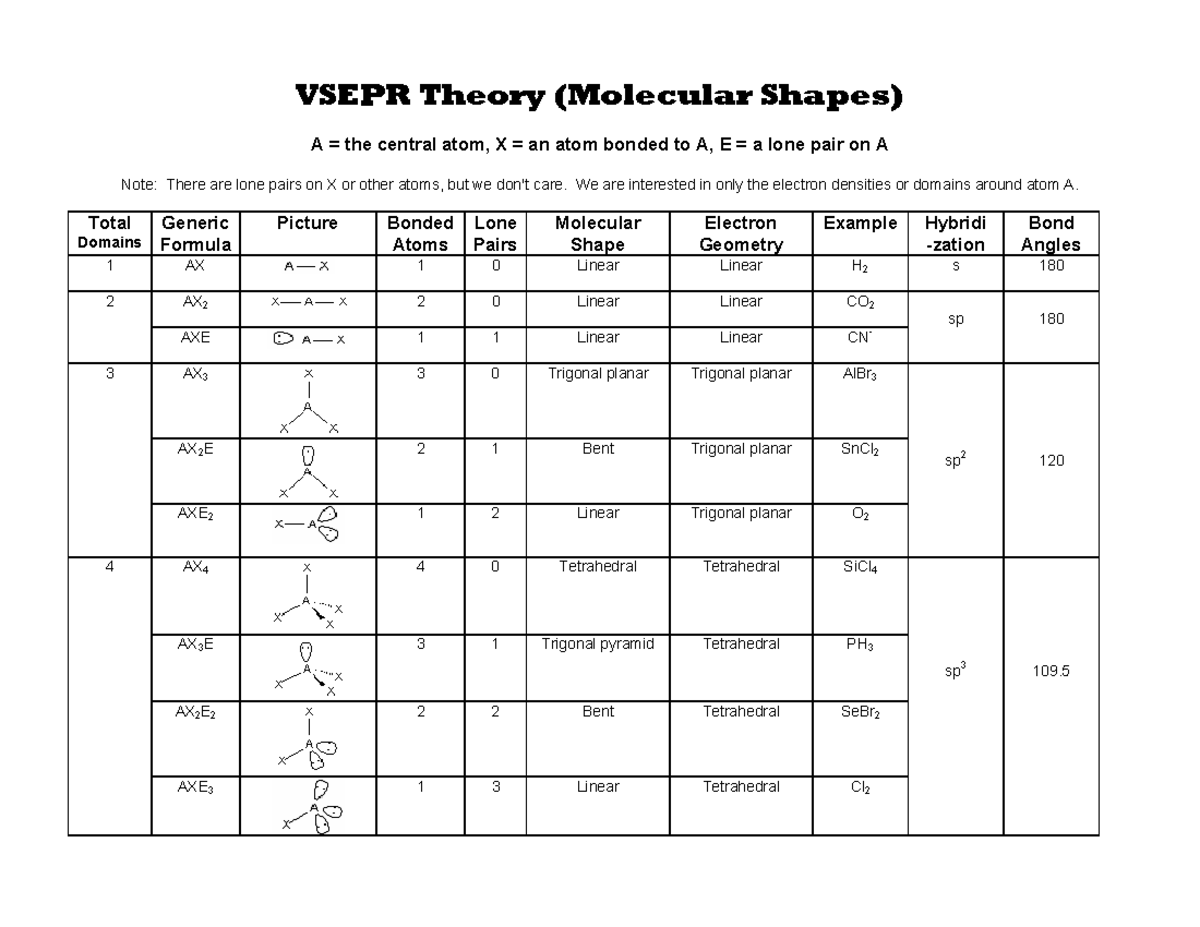
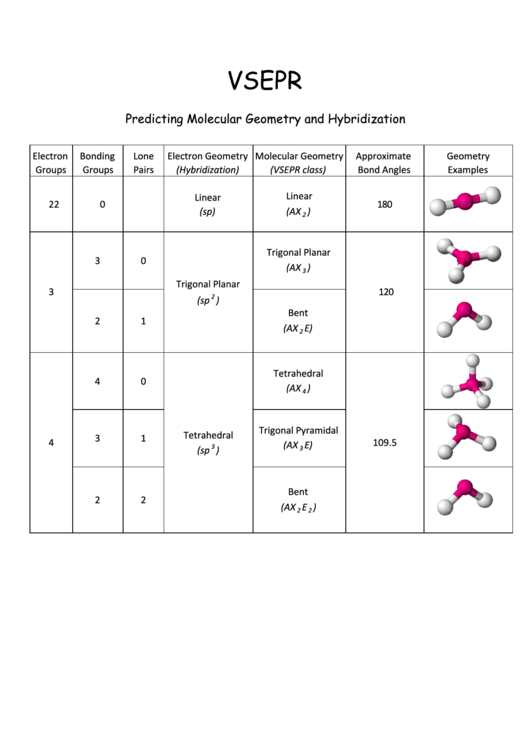
Vsepr Cheat Sheet
Vsepr Cheat Sheet - We are interested in only the electron densities or domains around atom a. There are lone pairs on x or other atoms, but we don't care. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: It is based on the assumption that pairs of electrons occupy space, and. A multiple bond (double bond or triple bond) counts as one electron group. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons.
Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. It is based on the assumption that pairs of electrons occupy space, and. We are interested in only the electron densities or domains around atom a. A multiple bond (double bond or triple bond) counts as one electron group. There are lone pairs on x or other atoms, but we don't care.
There are lone pairs on x or other atoms, but we don't care. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. A multiple bond (double bond or triple bond) counts as one electron group. It is based on the assumption that pairs of electrons occupy space, and. We are interested in only the electron densities or domains around atom a.
Vsepr Chart
A multiple bond (double bond or triple bond) counts as one electron group. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. We are interested in only the electron densities or domains around atom a. It is based on the assumption that pairs of electrons occupy space, and. There are.
Pin by Siphora Ketchakeu on organic chemistry 1 Teaching chemistry
Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: There are lone pairs on x or other atoms, but we don't care. We are interested in only the electron densities or domains around atom a. Web using vsepr to predict the shapes of molecules “electron groups” include bonds,.
Molecular geometry cheat sheet
We are interested in only the electron densities or domains around atom a. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: It is based on the assumption that pairs of electrons occupy space, and. There are lone pairs on x or other atoms, but we don't care..
Vsepr handout vesper cheat sheet VSEPR Theory (Molecular Shapes) A
Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: We are interested in only the electron densities or domains around atom a. It is based on the assumption that.
Química 12º 2.0 Tema 3 Enlace químico
It is based on the assumption that pairs of electrons occupy space, and. A multiple bond (double bond or triple bond) counts as one electron group. We are interested in only the electron densities or domains around atom a. There are lone pairs on x or other atoms, but we don't care. Web using vsepr to predict the shapes of.
Lewis structuresvsepr theory cheat sheet Vsepr Theory, Polyatomic Ion
Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: There are lone pairs on x or other atoms, but we don't care. It is based on the assumption that pairs of electrons occupy space, and. Web using vsepr to predict the shapes of molecules “electron groups” include bonds,.
XeO2F2 Lewis Structure, Geometry, Hybridization, and Polarity
A multiple bond (double bond or triple bond) counts as one electron group. We are interested in only the electron densities or domains around atom a. There are lone pairs on x or other atoms, but we don't care. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons. Web a.
PPT Covalent Bonding PowerPoint Presentation, free download ID590204
Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: A multiple bond (double bond or triple bond) counts as one electron group. There are lone pairs on x or other atoms, but we don't care. Web using vsepr to predict the shapes of molecules “electron groups” include bonds,.
Vsepr Theory Summary Chart Download Printable Pdf Templateroller Images
It is based on the assumption that pairs of electrons occupy space, and. There are lone pairs on x or other atoms, but we don't care. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: A multiple bond (double bond or triple bond) counts as one electron group..
Geometry Math geometry, Math cheat sheet, Basic math
We are interested in only the electron densities or domains around atom a. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: It is based on the assumption that pairs of electrons occupy space, and. A multiple bond (double bond or triple bond) counts as one electron group..
It Is Based On The Assumption That Pairs Of Electrons Occupy Space, And.
We are interested in only the electron densities or domains around atom a. Web a = the central atom, x = an atom bonded to a, e = a lone pair on a note: A multiple bond (double bond or triple bond) counts as one electron group. Web using vsepr to predict the shapes of molecules “electron groups” include bonds, lone pairs, and odd (unpaired) electrons.