Metal Oxides React With Water To Form
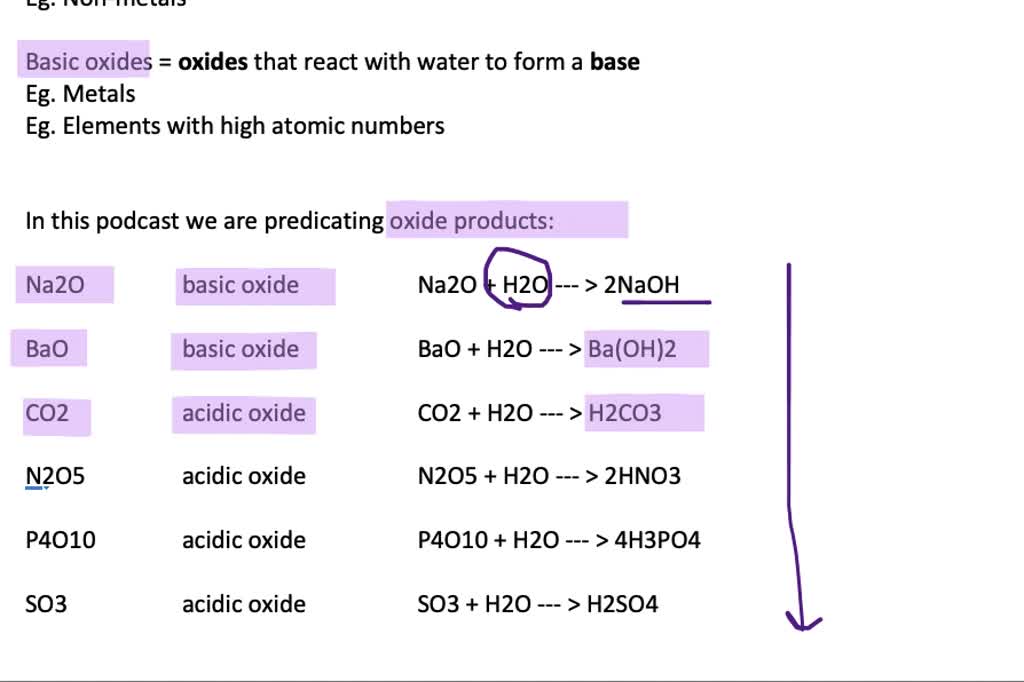
Metal Oxides React With Water To Form - (1) write an equation to show how potassium oxide reacts with water to form a base. While most metal oxides are insoluble in water, alkali metal and alkaline earth metal oxides are water soluble. Web metal oxides and metal hydroxides are basic in nature. If soluble in water, they react with water to produce hydroxides (alkalies) e.g., cao+ h 2o → ca(oh) 2 mgo+ h 2o →. Web basic oxides are the oxides of metals. Web some metal oxides are amphoteric because they can react with both acids and bases to produce salt and water. Web reactions of metals metals can react with water, acid and oxygen. Examples of such reactions are: If i add hcl to the hydroxide, can i be sure. Here metal displaces hydrogen in acid and forms a salt.
Web metal oxides’ interaction with water. Here metal displaces hydrogen in acid and forms a salt. (1) write an equation to show how potassium oxide reacts with water to form a base. Web the reaction of metal oxides with water. If soluble in water, they react with water to produce hydroxides (alkalies) e.g., cao+ h 2o → ca(oh) 2 mgo+ h 2o →. Web metal oxides and metal hydroxides are basic in nature. Web basic metal oxides at a low oxidation state react with aqueous acids to form solutions of salts and water. This is because of the unique nature and bonding in the oxide. Web reaction with water: Some elements in the center of the periodic table form amphoteric oxides, which will react.
So they react with acid to form salt and water. Some elements in the center of the periodic table form amphoteric oxides, which will react. Web basic oxides are the oxides of metals. Na 2 o ( s ) + h 2 o ( l ) naoh ( a q ). (1) write an equation to show how potassium oxide reacts with water to form a base. This is because of the unique nature and bonding in the oxide. Web metal oxides’ interaction with water. If i add hcl to the hydroxide, can i be sure. Sodium oxide, na 2 o, and magnesium oxide, mgo, are made up of ions. Metal oxides (to the left of the periodic table):
Nature of metal oxides Metals and Non metals Chemistry Khan
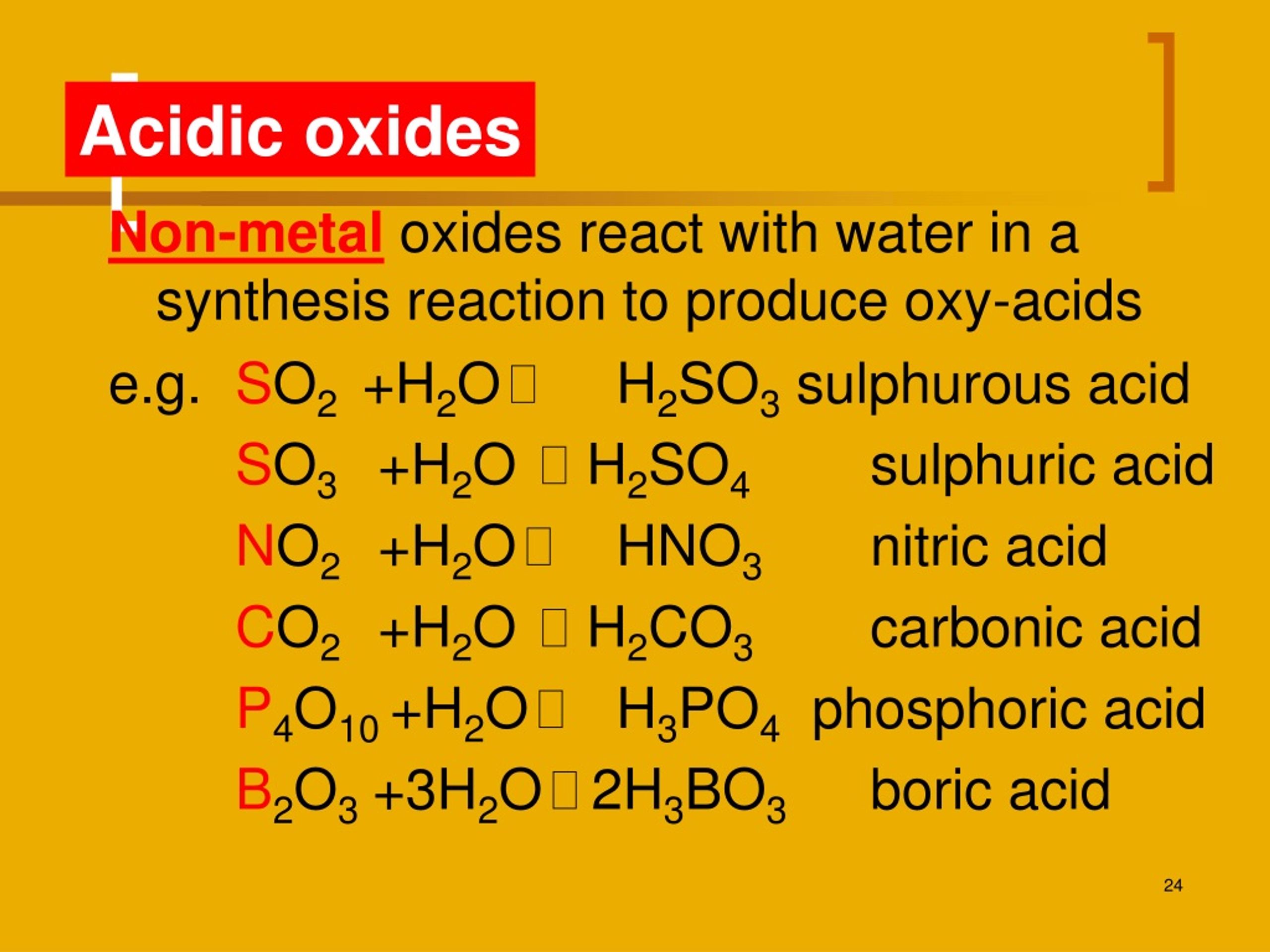
Nonmetals generally form acidic oxides. Web science chemistry chemistry questions and answers metal oxides can react with water to form bases. Web explanations (including important chemical equations): The reactivity of the metal determines which reactions the metal participates in. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.
[MCQ] Which of the statements is not correct? All metal oxides react
Web metal oxides’ interaction with water. Web some metal oxides are amphoteric because they can react with both acids and bases to produce salt and water. Web basic oxides are the oxides of metals. If i add hcl to the hydroxide, can i be sure. If soluble in water, they react with water to produce hydroxides (alkalies) e.g., cao+ h.
PPT Chemical Equations and Reactions PowerPoint Presentation, free
Some elements in the center of the periodic table form amphoteric oxides, which will react. So they react with acid to form salt and water. Web basic oxides are the oxides of metals. Now here is my question: Here metal displaces hydrogen in acid and forms a salt.
PPT Working With Chemical Reactions PowerPoint Presentation ID3661856
Most of the metal oxides are insoluble in water, but oxides of alkali metals and alkaline earth metals are soluble in water. Web as far as i know, only metal oxides from group 1 and 2 react in water, making hydroxides. Web basic oxides are the oxides of metals. While most metal oxides are insoluble in water, alkali metal and.
Metal oxides can react with water to form bases.
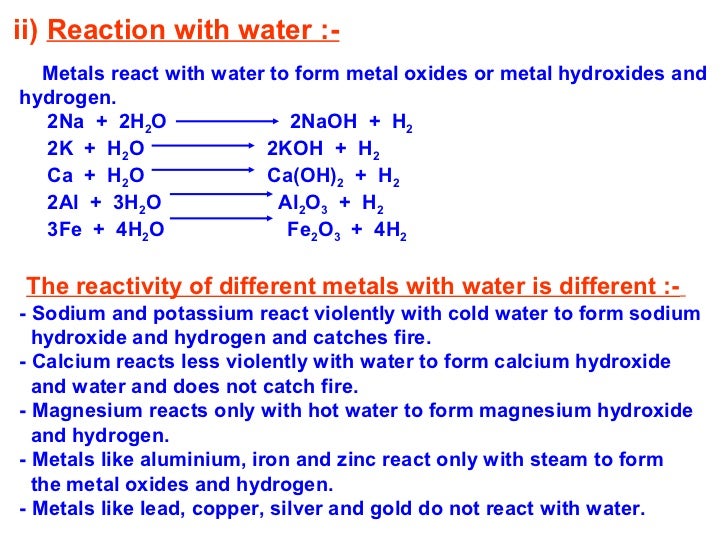
Here metal displaces hydrogen in acid and forms a salt. A concentrated solution of sodium oxide in water. For example, magnesium oxide reacts with water. Web the oxides of the metals of groups 1 and 2 and of thallium(i) oxide react with water and form hydroxides. Examples include the reaction of cobalt(ii) oxide.
Difference Between Metal Oxides and Non Metal Oxides Definition
So they react with acid to form salt and water. (1) write an equation to show how potassium oxide reacts with water to form a base. Web metals generally form basic oxides. Web some metal oxides are amphoteric because they can react with both acids and bases to produce salt and water. Web science chemistry chemistry questions and answers metal.
Metals and non metals
If i add hcl to the hydroxide, can i be sure. This is because of the unique nature and bonding in the oxide. A concentrated solution of sodium oxide in water. Web basic oxides are the oxides of metals. Web the reaction of metal oxides with water.
[MCQ] Which of the statements is not correct? All metal oxides react
Examples of such reactions are: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. While most metal oxides are insoluble in water, alkali metal and alkaline earth metal oxides are water soluble. Web basic oxides are the oxides of metals. Web the oxides of the metals of groups 1 and 2 and of thallium(i) oxide react with.
Oxides RASILL
Most of the metal oxides are insoluble in water, but oxides of alkali metals and alkaline earth metals are soluble in water. A concentrated solution of sodium oxide in water. If soluble in water, they react with water to produce hydroxides (alkalies) e.g., cao+ h 2o → ca(oh) 2 mgo+ h 2o →. So they react with acid to form.
How acids react with metallic oxides CBSE Class 10 Chemistry Notes
Most of the metal oxides are insoluble in water, but oxides of alkali metals and alkaline earth metals are soluble in water. Metal oxides that dissolve in water react with water to form basic solutions. Metal oxides can react with water to form bases. Sodium oxide, na 2 o, and magnesium oxide, mgo, are made up of ions. Watch this.
Web Metal Oxides’ Interaction With Water.
A concentrated solution of sodium oxide in water. Web basic metal oxides at a low oxidation state react with aqueous acids to form solutions of salts and water. Web the reaction of metal oxides with water. Web metal oxides and metal hydroxides are basic in nature.
While Most Metal Oxides Are Insoluble In Water, Alkali Metal And Alkaline Earth Metal Oxides Are Water Soluble.
Web as far as i know, only metal oxides from group 1 and 2 react in water, making hydroxides. Web easy solution verified by toppr correct option is a) metals react with water to form metal oxides or metal hydroxide (froming an alkaline solution) and hydrogen gas. Most of the metal oxides are insoluble in water, but oxides of alkali metals and alkaline earth metals are soluble in water. Web metals generally form basic oxides.
Metal Oxides Can React With Water To Form Bases.
If soluble in water, they react with water to produce hydroxides (alkalies) e.g., cao+ h 2o → ca(oh) 2 mgo+ h 2o →. Examples of such reactions are: Web reaction with water: Nonmetals generally form acidic oxides.
So They React With Acid To Form Salt And Water.
Web basic oxides are the oxides of metals. Web explanations (including important chemical equations): Metal oxides that dissolve in water react with water to form basic solutions. (1) write an equation to show how barium oxide reacts with water to form.

![[MCQ] Which of the statements is not correct? All metal oxides react](https://d1avenlh0i1xmr.cloudfront.net/large/0146fde4-9748-4104-875a-ccbefb146ee1/reaction-of-metal-carbonate-with-acid---teachoo-01.jpg)


![[MCQ] Which of the statements is not correct? All metal oxides react](https://d1avenlh0i1xmr.cloudfront.net/1942b2cf-3ca7-4b5e-9607-79be588ba41c/reaction-of-non-metals-oxides-with-water---teachoo.jpg)

