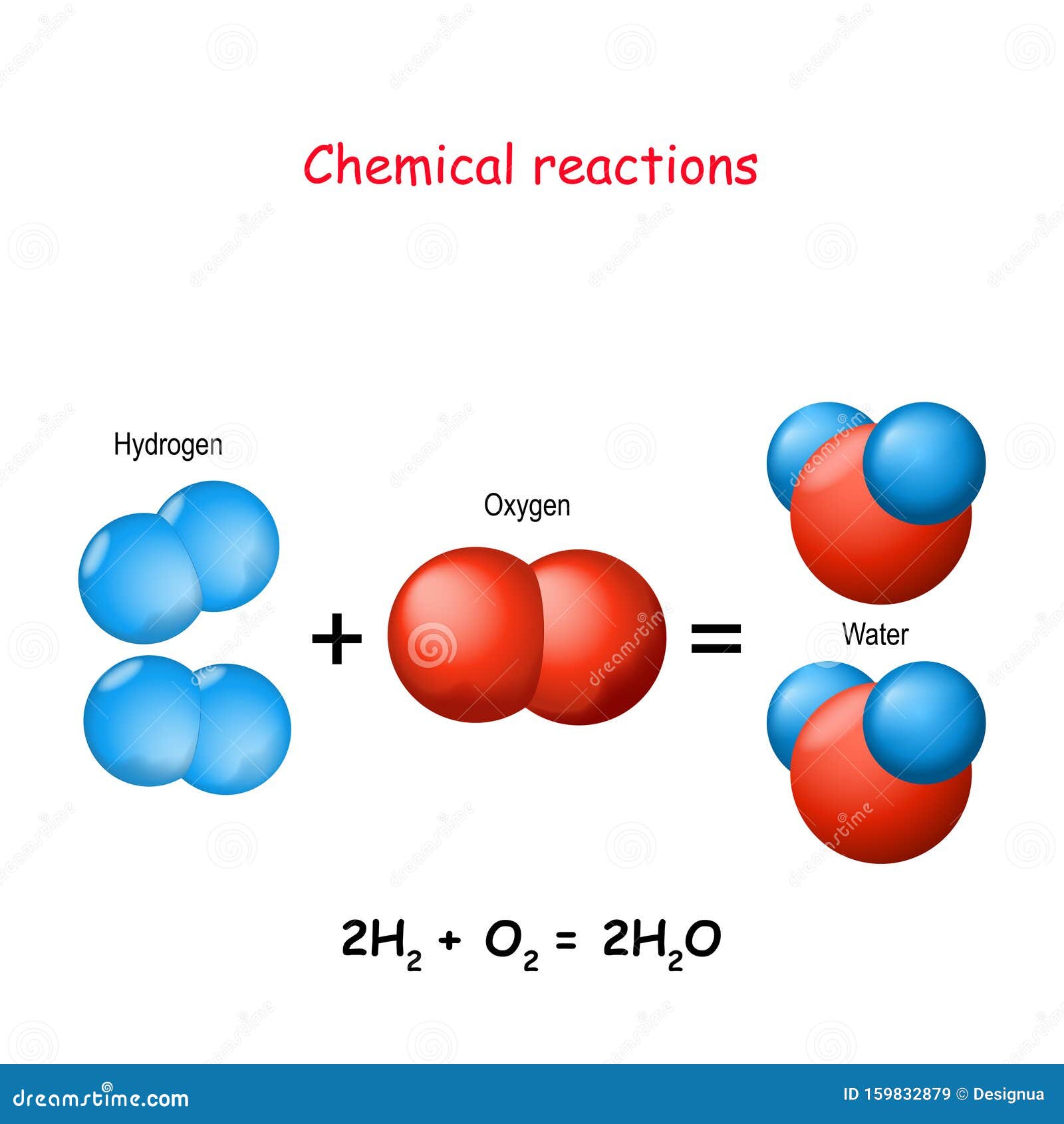
Hydrogen And Oxygen React To Form Water
Hydrogen And Oxygen React To Form Water - The reaction between hydrogen and oxygen to form water is represented by the. H 2(g) + 1 2o2(g) → h 2o(l) clearly, dihydrogen must be present in a 2:1 molar ratio with respect to dioxygen. Web oxygen reacts with hydrogen to produce two compounds: Web we need a stoichiometric equation for water synthesis: To produce two molecules of water. Web in this case, the enthalpy of 484 kj 484 k j is released when 2 mol 2 m o l of hydrogen gas react with 1 mol 1 m o l of oxygen gas to form 2 mol 2 m o l of gaseous water: In english, the equation says: Web hydrogen gas reacts with oxygen gas to form liquid water. Web 10/05/2019 chemistry high school answered • expert verified hydrogen and oxygen react chemically to form water how much water would form if 14.8grams of. 8 points hydrogen and oxygen react to form water (see the.
2h 2 (g) + o 2 (g) ==> 2h. In english, the equation says: Web 10/05/2019 chemistry high school answered • expert verified hydrogen and oxygen react chemically to form water how much water would form if 14.8grams of. Web chemical equations chemical reactions are described by chemical equations. Therefore, 3g of hydrogen gas will require =. Web to enable a future society based on sun and wind energy, transforming electricity into chemical energy in the form of fuels is crucial. It weakens the bond between carbon and sulfur by repelling the electrons even more toward sulfur, and it. Web the actual reaction to make water is a bit more complicated: 190k views 9 years ago physical science. Web the heat allows the hydrogen to combine with the small amount of oxygen in the air.
Water ( h 2 o) and hydrogen peroxide ( h 2 o 2 ). Web hydrogen and oxygen can form compounds other than diatomic gasses and water also. When you look in the test tube afterwards there will be water condensed on the glass! 190k views 9 years ago physical science. To produce two molecules of water. Web tutor 5.0 (141) ph.d. Grant mason from the byu department of physics and astronomy demonstrates the reaction of. 2h2 + o2 = 2h2o + energy. Thus in this reaction, two moles of. In english, the equation says:
Free Online Help When hydrogen sulfide gas , H2S react with oxygen
The reaction between hydrogen and oxygen to form water is represented by the. How much water would be. H 2(g) + 1 2o2(g) → h 2o(l) clearly, dihydrogen must be present in a 2:1 molar ratio with respect to dioxygen. Web hydrogen gas reacts with oxygen gas to form liquid water. Web to enable a future society based on sun.
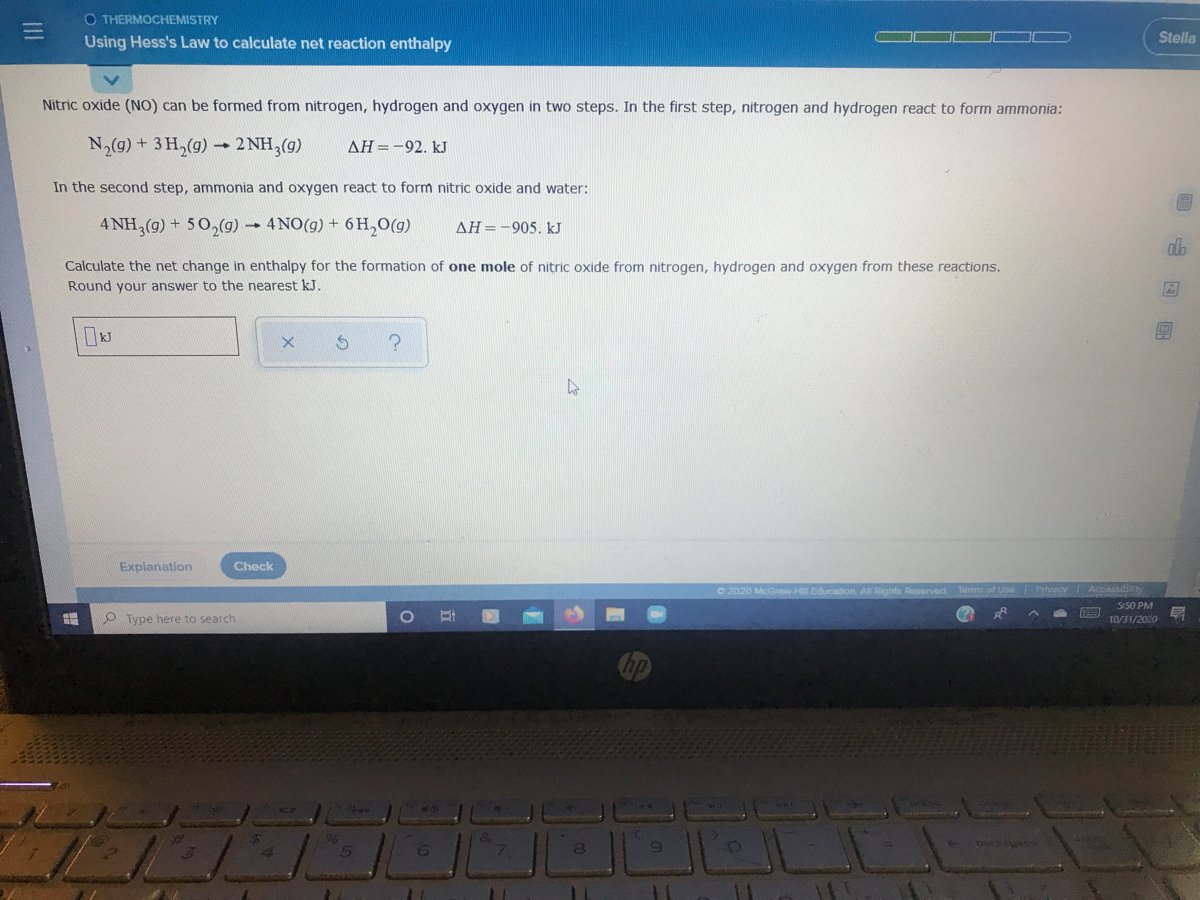
Answered Nitric oxide (NO) can be formed from… bartleby
Therefore, 3g of hydrogen gas will require =. Web in this case, the enthalpy of 484 kj 484 k j is released when 2 mol 2 m o l of hydrogen gas react with 1 mol 1 m o l of oxygen gas to form 2 mol 2 m o l of gaseous water: Web oxygen reacts with hydrogen to.
4 moles of hydrogen react with 5 moles of oxygen to form water
Web hydrogen and oxygen can form compounds other than diatomic gasses and water also. 2h2 + o2 = 2h2o + energy. It weakens the bond between carbon and sulfur by repelling the electrons even more toward sulfur, and it. Web when you burn hydrogen and oxygen gas, the reaction is: When you look in the test tube afterwards there will.
Water Molecule. Oxygen and Hydrogen Stock Vector Illustration of
Web chemical equations chemical reactions are described by chemical equations. 2h 2 (g) + o 2 (g) ==> 2h. Web the actual reaction to make water is a bit more complicated: Water is a versatile compound and participates in acid. Web solution hydrogen and oxygen combine in a ratio of 1:8 means that 1g of hydrogen gas requires 8g of.
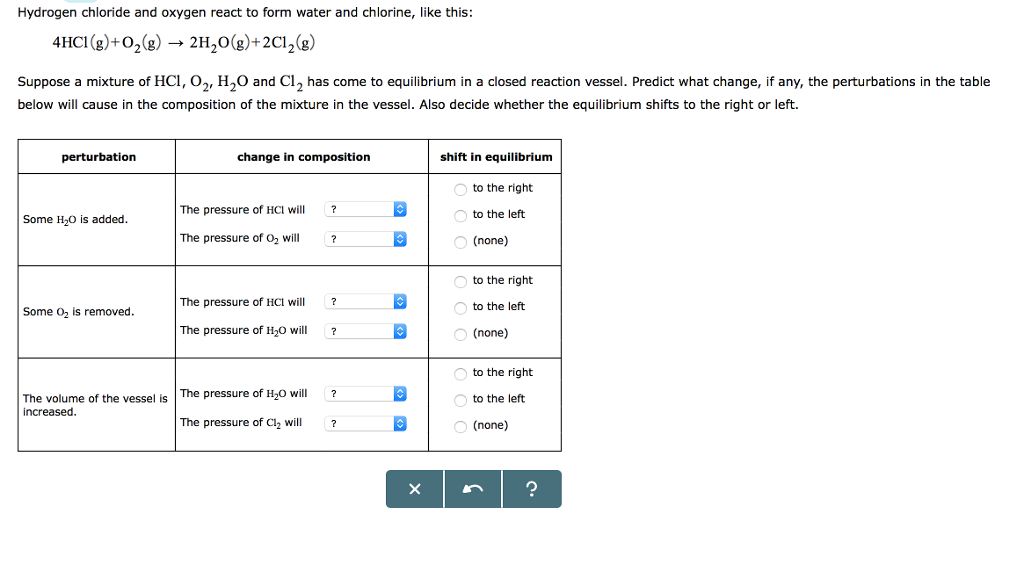
Solved Hydrogen chloride and oxygen react to form water and
Thus in this reaction, two moles of. Web solution hydrogen and oxygen combine in a ratio of 1:8 means that 1g of hydrogen gas requires 8g of oxygen gas to form water. To produce two molecules of water. Web hydrogen and oxygen can form compounds other than diatomic gasses and water also. It weakens the bond between carbon and sulfur.
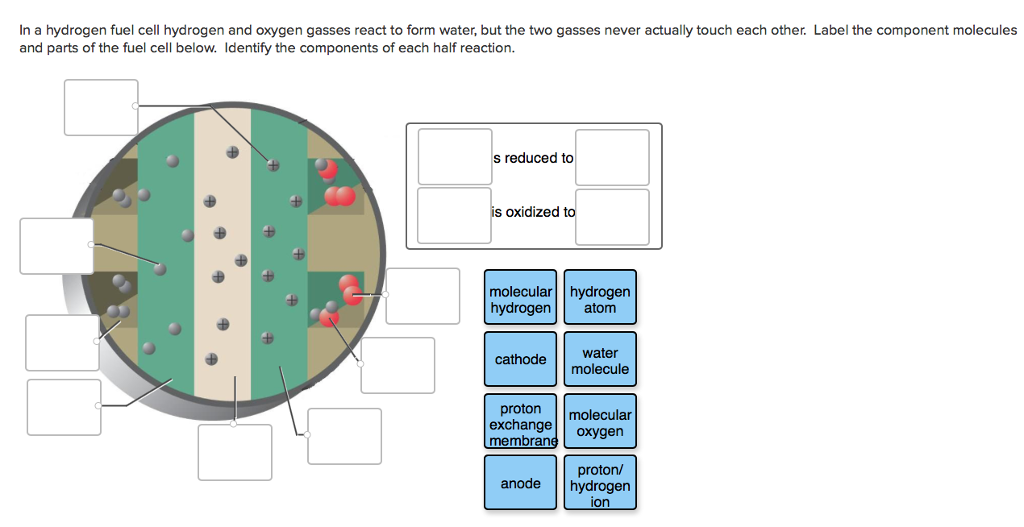
Solved In a hydrogen fuel cell hydrogen and oxygen gasses
Web hydrogen and oxygen can form compounds other than diatomic gasses and water also. Web this partial bond formation has two effects: Web tutor 5.0 (141) ph.d. Web when you burn hydrogen and oxygen gas, the reaction is: In english, the equation says:
Solved Hydrogen gas reacts with oxygen gas to produce water.
Web to enable a future society based on sun and wind energy, transforming electricity into chemical energy in the form of fuels is crucial. Web hydrogen and oxygen can form compounds other than diatomic gasses and water also. Hydrogen and oxygen react chemically to form water. In english, the equation says: To produce two molecules of water.
Reaction of Hydrogen and Oxygen to water Stock Vector Image & Art Alamy
8 points hydrogen and oxygen react to form water (see the. H 2(g) + 1 2o2(g) → h 2o(l) clearly, dihydrogen must be present in a 2:1 molar ratio with respect to dioxygen. Therefore, 3g of hydrogen gas will require =. Web to enable a future society based on sun and wind energy, transforming electricity into chemical energy in the.
6.(a) Hydrogen and oxygen combine in the ratio of 18 by mass to form
Web this partial bond formation has two effects: H 2(g) + 1 2o2(g) → h 2o(l) clearly, dihydrogen must be present in a 2:1 molar ratio with respect to dioxygen. Web to enable a future society based on sun and wind energy, transforming electricity into chemical energy in the form of fuels is crucial. To produce two molecules of water..
How to Make Oxygen and Hydrogen from Water Using Electrolysis
The compound h 2 o 2 , called hydrogen peroxide, is less stable than water, but can be. Web hydrogen and oxygen can form compounds other than diatomic gasses and water also. The reaction between hydrogen and oxygen to form water is represented by the. Web the actual reaction to make water is a bit more complicated: Water ( h.
Web The Actual Reaction To Make Water Is A Bit More Complicated:
Web hydrogen gas reacts with oxygen gas to form liquid water. Web when you burn hydrogen and oxygen gas, the reaction is: 8 points hydrogen and oxygen react to form water (see the. How much water would be.
To Produce Two Molecules Of Water.
The compound h 2 o 2 , called hydrogen peroxide, is less stable than water, but can be. Water ( h 2 o) and hydrogen peroxide ( h 2 o 2 ). Web in this case, the enthalpy of 484 kj 484 k j is released when 2 mol 2 m o l of hydrogen gas react with 1 mol 1 m o l of oxygen gas to form 2 mol 2 m o l of gaseous water: Web hydrogen and oxygen can form compounds other than diatomic gasses and water also.
Water Is A Versatile Compound And Participates In Acid.
Thus in this reaction, two moles of. Web oxygen reacts with hydrogen to produce two compounds: Web we need a stoichiometric equation for water synthesis: Grant mason from the byu department of physics and astronomy demonstrates the reaction of.
2H2 + O2 = 2H2O + Energy.
Hydrogen and oxygen react chemically to form water. Therefore, 3g of hydrogen gas will require =. In english, the equation says: When you look in the test tube afterwards there will be water condensed on the glass!