How To Read A 10 Ml Graduated Cylinder
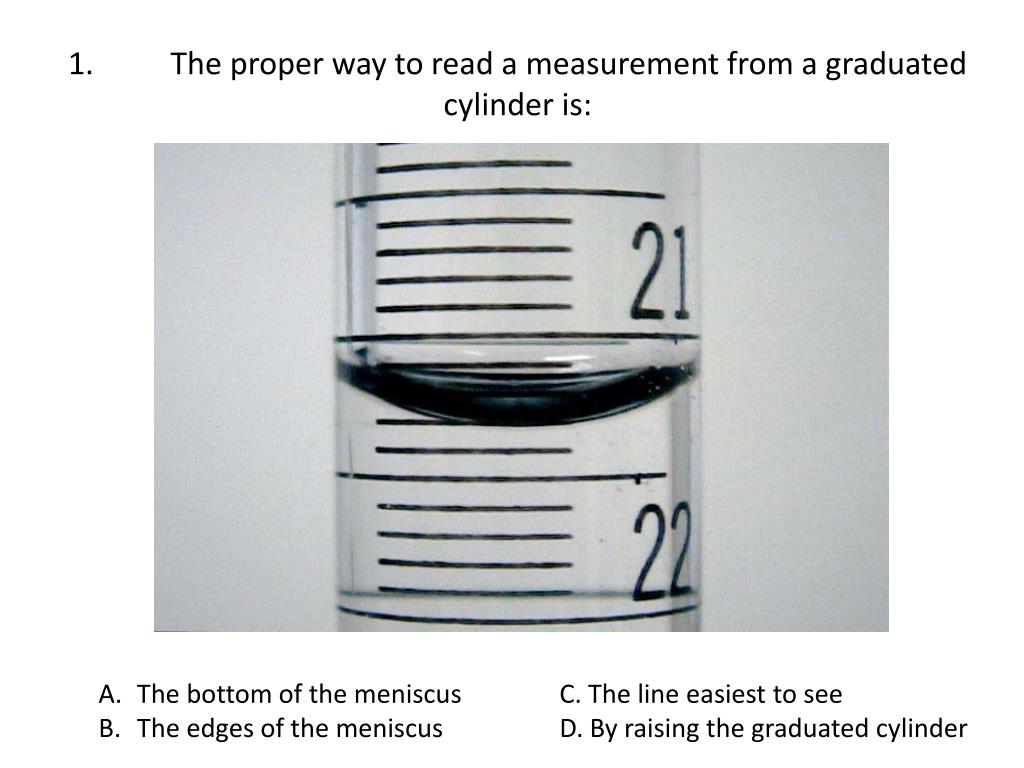
How To Read A 10 Ml Graduated Cylinder - Web the uncertain digit (the last digit of the reading) is estimated. Quantitative data involves numbers, such as the. Web one graduation therefore = 1/10 ml, or here subtract 6 from 7 (answer = 1) and count that there 10 graduations between the 6 and 7 labelled graduations. What's the meaning of dwag? Web an easy way to find out is to fill the 100ml graduated cylinder exactly halfway, i.e. To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 ml marks into tenths of a milliliter, and then make a reading (estimate) at the. Remember that space equals volume. A graduation is 0.5 ml. Qualitative data collecting data is an important part of any scientific investigation. Web reading a graduated cylinder starts with dividing the difference between adjacent numbered lines by the number of unmarked lines counted from one numbered line to the next.
Accepts a small range of estimates for the final digit in the reading. Is a 10 ml or 50 ml graduated cylinder. If the cylinder is too large to fit in your hand, it may be a good idea to buy a smaller one. For mercury, take the measurement from the top of the. A graduation is 0.5 ml. Web how to use a graduated cylinder. You can also use a ruler to measure the diameter of a cylinder. Web transfer pellets to the beaker weighed in the previous step, and measure the mass of the beaker and pellets together. Web reading a graduated cylinder starts with dividing the difference between adjacent numbered lines by the number of unmarked lines counted from one numbered line to the next. If you are unsure of the size of your cylinder, you can measure it with a caliper.
If you are unsure of the size of your cylinder, you can measure it with a caliper. Qualitative data collecting data is an important part of any scientific investigation. Web the uncertain digit (the last digit of the reading) is estimated. Web 15 ml — 10 ml = 5 ml count the number of spaces between the 2 graduations. A graduation is 0.5 ml. Empty the water into a clean beaker. Place the cylinder on the weighing balance. To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 ml marks into tenths of a milliliter, and then make a reading (estimate) at the. If the cylinder is too large to fit in your hand, it may be a good idea to buy a smaller one. 5 ml/10 spaces = 0.5 ml per space the answer tells you the value between each marked graduation on the cylinder.
25 ml glass graduated cylinder, glass
Tare the balance to make the reading zero. Accepts a small range of estimates for the final digit in the reading. What's the meaning of dwag? Web 15 ml — 10 ml = 5 ml count the number of spaces between the 2 graduations. Web how to use a graduated cylinder.
Best 10 Ml Graduated Cylinder out of top 19 in 2020
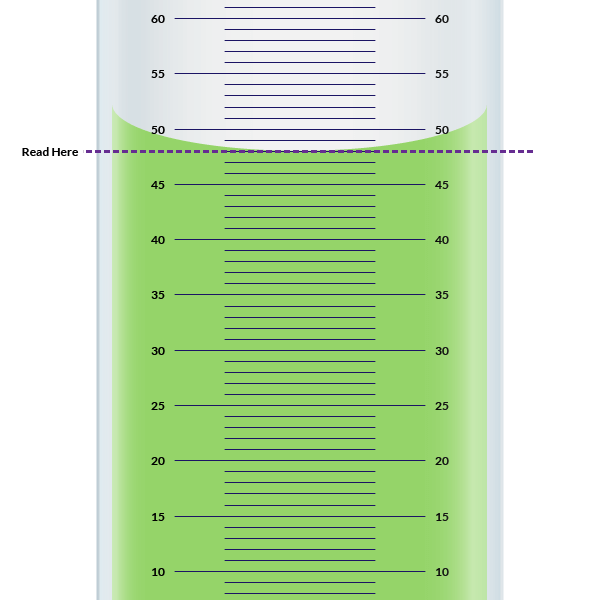
If there were no air, it would fill the graduated cylinder to the 100ml line. Is a 10 ml or 50 ml graduated cylinder. For water and most liquids, this is the bottom of the meniscus. Depending on the size of the graduated cylinder and the graduations, the uncertain digit may be to the milliliter ( 1x ), the tenth.
Measurement Uncertainty, Accuracy, and Precision Chemistry I
Place the cylinder on the weighing balance. To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 ml marks into tenths of a milliliter, and then make a reading (estimate) at the. You can also use a ruler to measure the diameter of a cylinder. Therefore, the scale increment.
Reading A Graduated Cylinder slidesharedocs
Web reading a graduated cylinder starts with dividing the difference between adjacent numbered lines by the number of unmarked lines counted from one numbered line to the next. For water and most liquids, this is the bottom of the meniscus. Empty the water into a clean beaker. If there were no air, it would fill the graduated cylinder to the.
50 mL Graduated Cylinder for Measuring Home Science Tools
Empty the water into a clean beaker. Web 15 ml — 10 ml = 5 ml count the number of spaces between the 2 graduations. Look straight across the meniscus, reading. Web how to use a graduated cylinder. Remember that space equals volume.
10 mL Graduated Cylinder for Measuring Home Science Tools
Accepts a small range of estimates for the final digit in the reading. Look straight across the meniscus, reading. Web measure so that the line you are reading is even with the center of the meniscus. Tare the balance to make the reading zero. What are the lines on a graduated cylinder?
1000 ml Graduated Cylinder polypropylene
Checks accuracy of measurement and provides feedback. Empty the water into a clean beaker. Accepts a small range of estimates for the final digit in the reading. Qualitative data collecting data is an important part of any scientific investigation. Pour distilled water into the cylinder, up to 25% of its total capacity (be careful to measure the exact volume).
VOS 10ml Graduated Measuring Cylinder Borosilicate Glass BCG Film
Web one graduation therefore = 1/10 ml, or here subtract 6 from 7 (answer = 1) and count that there 10 graduations between the 6 and 7 labelled graduations. To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 ml marks into tenths of a milliliter, and then make.
PLASTIC GRADUATED MEASURING CYLINDER 10 ML Science2Life
Then pour the sand in. The correct reading is 30.0 ml… A graduation is 0.5 ml. If the cylinder is too large to fit in your hand, it may be a good idea to buy a smaller one. Next, count that there are ten intervals between the labeled graduations.
Cheap Graduated Cylinder 10ml, find Graduated Cylinder 10ml deals on
Quantitative data involves numbers, such as the. You can also use a ruler to measure the diameter of a cylinder. Place the cylinder on the weighing balance. If there were no air, it would fill the graduated cylinder to the 100ml line. Checks accuracy of measurement and provides feedback.
Quantitative Data Involves Numbers, Such As The.
Checks accuracy of measurement and provides feedback. Web transfer pellets to the beaker weighed in the previous step, and measure the mass of the beaker and pellets together. Web 15 ml — 10 ml = 5 ml count the number of spaces between the 2 graduations. Therefore, the scale increment is 2 ml/10 graduations = 0.2 ml/graduation.
If The Cylinder Is Too Large To Fit In Your Hand, It May Be A Good Idea To Buy A Smaller One.
Web the graduated cylinders are always read to 2 decimal places. What's the meaning of dwag? Web the uncertain digit (the last digit of the reading) is estimated. Empty the water into a clean beaker.
What Are The Lines On A Graduated Cylinder?
Place the cylinder on the weighing balance. A graduation is 0.5 ml. Depending on the size of the graduated cylinder and the graduations, the uncertain digit may be to the milliliter ( 1x ), the tenth of a milliliter ( 1.x. Find the center of the meniscus.
Pour Distilled Water Into The Cylinder, Up To 25% Of Its Total Capacity (Be Careful To Measure The Exact Volume).
Web measure so that the line you are reading is even with the center of the meniscus. Qualitative data collecting data is an important part of any scientific investigation. Then pour the sand in. Tare the balance to make the reading zero.