How To Know How Many Bonds An Element Can Form
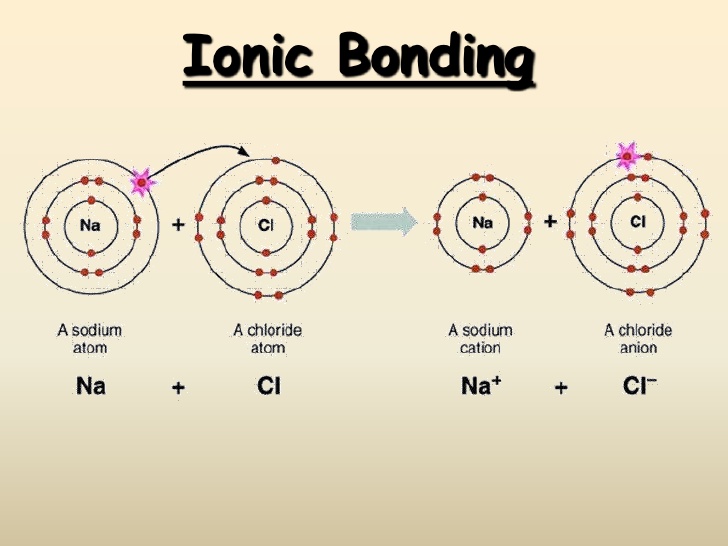
How To Know How Many Bonds An Element Can Form - Web best answer copy by which group (or column) it's in. The number of covalent bonds is equal to eight minus the group number. It's named a covalent bond. The first way gives rise to what is called an ionic bond. For example, in methane (ch 4 _4 4 start subscript, 4, end subscript), carbon forms covalent bonds with. Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since. Web there are three basic ways that the outer electrons of atoms can form bonds: Consider as an example an atom of sodium,. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. This is summarized in the table below.
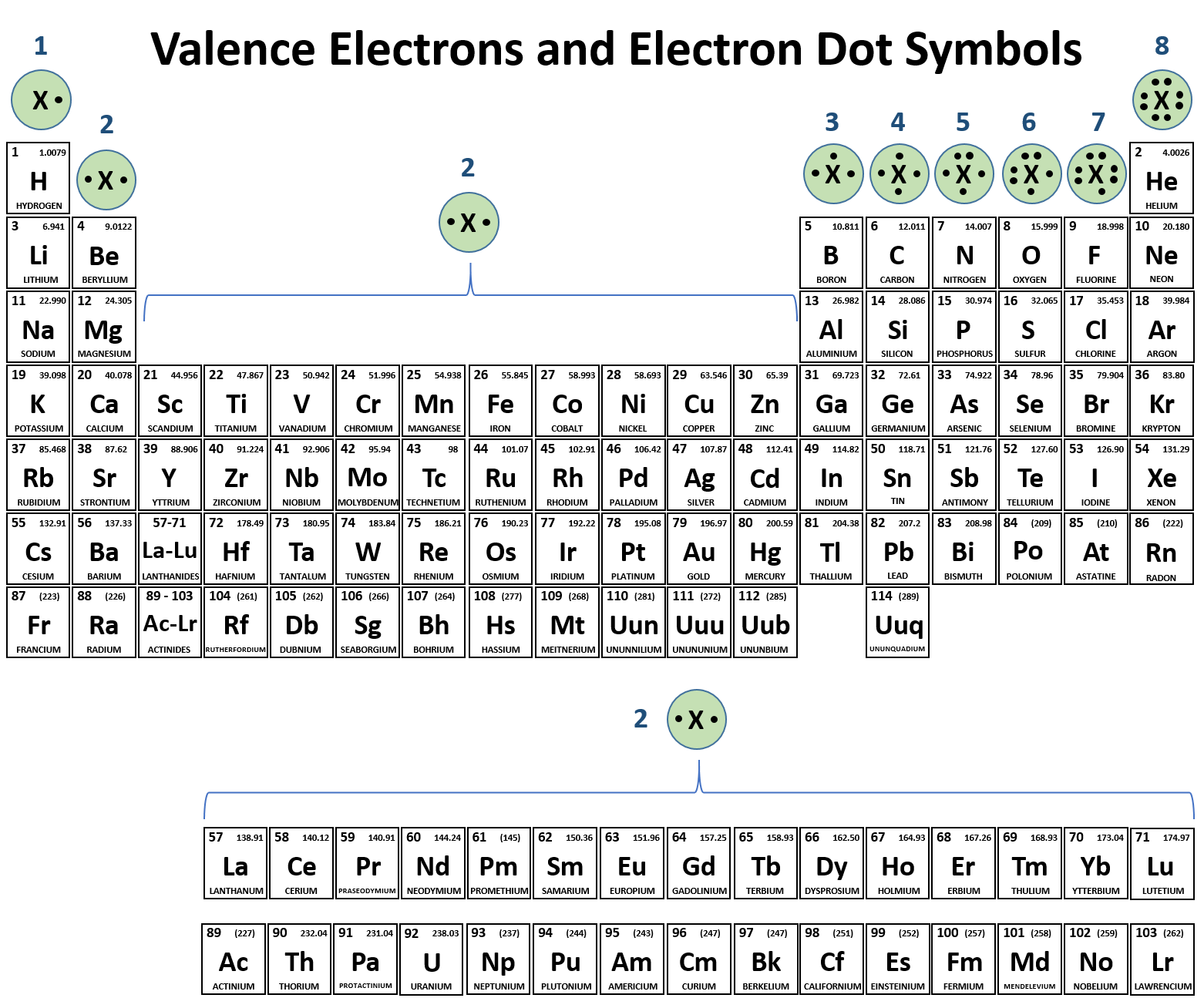
The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. Web how can you tell how many bonds an atom can form? The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. Web there is a quick way to work out how many covalent bonds an element will form. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. The table below shows the number of bonds formed by elements in groups. Web carbon atoms may thus form bonds to as many as four other atoms. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web best answer copy by which group (or column) it's in. The first way gives rise to what is called an ionic bond.
The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. Consider as an example an atom of sodium,. The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. 4 to 7 (iupac groups 14 to 17). Web carbon atoms may thus form bonds to as many as four other atoms. For example, in methane (ch 4 _4 4 start subscript, 4, end subscript), carbon forms covalent bonds with. Web how can you tell how many bonds an atom can form? The first way gives rise to what is called an ionic bond. The single place digit refers to the number of electrons in the valence shell of the elements in that group, with. Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since.
What are similarities of covalent and ionic bonding? Socratic
Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. Web best answer copy by which group (or.
Elements and Chemical Bonds
Web there is a quick way to work out how many covalent bonds an element will form. In a covalent bond, the stability of the bond comes from the shared electrostatic attraction between the two. Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration.
Elements and Chemical Bonds
The single place digit refers to the number of electrons in the valence shell of the elements in that group, with. The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. In a covalent bond, the stability of the bond comes from the shared electrostatic.
11 Types of scientific changes with examples
The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. Web carbon atoms may thus form bonds to as many as four other atoms. Web the number of covalent bonds that an atom can form depends on the number of available electrons found in.
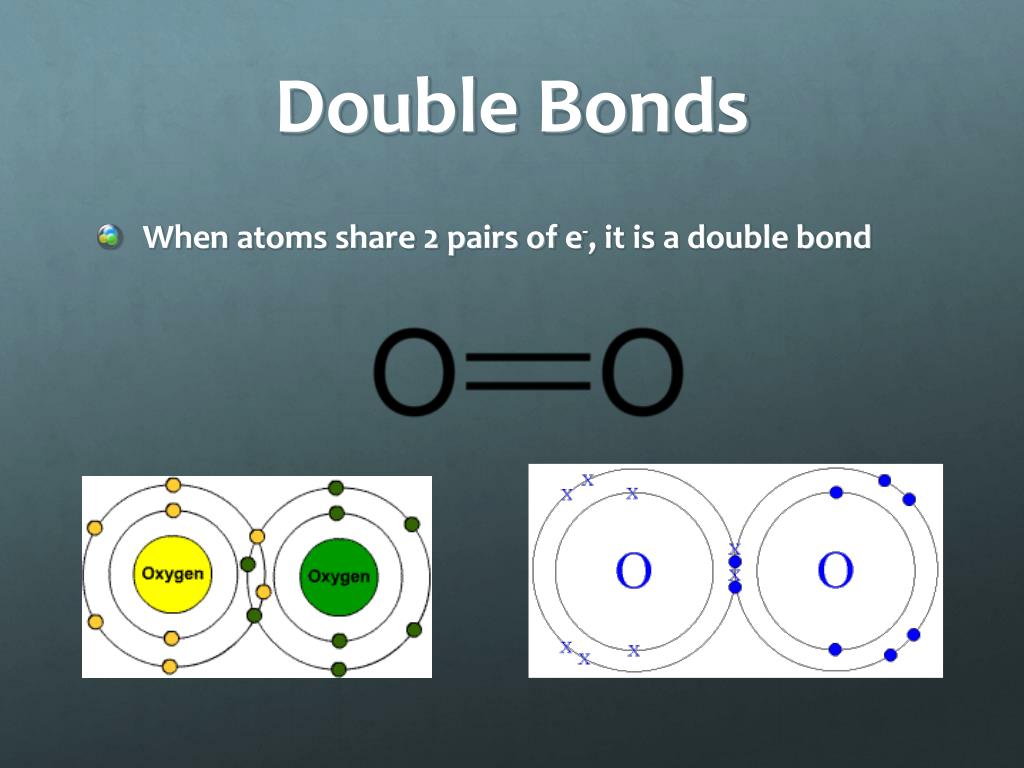
Single, Double, and Triple Bonds
Web there is a quick way to work out how many covalent bonds an element will form. The columns of the periodic table, which contain elements that show a family resemblance, are called. The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. Web.
Covalent Bonds Biology for NonMajors I
4 to 7 (iupac groups 14 to 17). Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since. The columns of the periodic table, which contain elements that show a family resemblance, are called. Web best answer copy by which group (or.
How to Predict number of bonds each element forms ChemSimplified
Web a covalent bond is formed when two atoms share electron pairs. This is summarized in the table below. 4 to 7 (iupac groups 14 to 17). The columns of the periodic table, which contain elements that show a family resemblance, are called. It's named a covalent bond.
PPT Chapter 22 Chemical Bonding PowerPoint Presentation, free
The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. The single place digit refers to the number of electrons in the valence shell of the elements in that group, with. The columns of the periodic table, which contain elements that show a family resemblance,.
How to Predict number of bonds each element forms ChemSimplified
The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. For example, in methane (ch 4 _4 4 start subscript, 4, end subscript), carbon forms covalent bonds with. Web carbon atoms may thus form bonds to as many as four other atoms. In a.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Web for most elements, a full outer shell is eight electrons. The single place digit refers to the number of electrons in the valence shell of the elements in that group, with. Consider as an example an atom of sodium,. Web how can you tell how many bonds an atom can form? The first way gives rise to what is.
It's Named A Covalent Bond.
The single place digit refers to the number of electrons in the valence shell of the elements in that group, with. The first way gives rise to what is called an ionic bond. Web the number of covalent bonds that an atom can form depends on the number of available electrons found in its outermost (valence) shell. Web the valency of an element tells us how much atoms do the atom of that particular element needs to achieve a stable electronic configuration so, here since.
In A Covalent Bond, The Stability Of The Bond Comes From The Shared Electrostatic Attraction Between The Two.
Web a covalent bond is formed when two atoms share electron pairs. The table below shows the number of bonds formed by elements in groups. For example, in methane (ch 4 _4 4 start subscript, 4, end subscript), carbon forms covalent bonds with. Web carbon atoms may thus form bonds to as many as four other atoms.
The Number Of Covalent Bonds Is Equal To Eight Minus The Group Number.
The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states. The columns of the periodic table, which contain elements that show a family resemblance, are called. 4 to 7 (iupac groups 14 to 17). Web there is a quick way to work out how many covalent bonds an element will form.
Consider As An Example An Atom Of Sodium,.
Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. Web best answer copy by which group (or column) it's in. The number of bonds for a neutral atom is equal to the number of electrons in the full valence shell (2 or 8 electrons) minus. This is summarized in the table below.