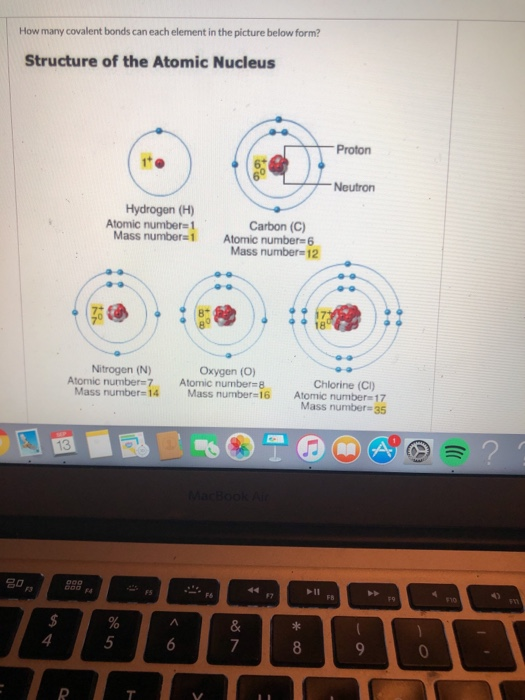
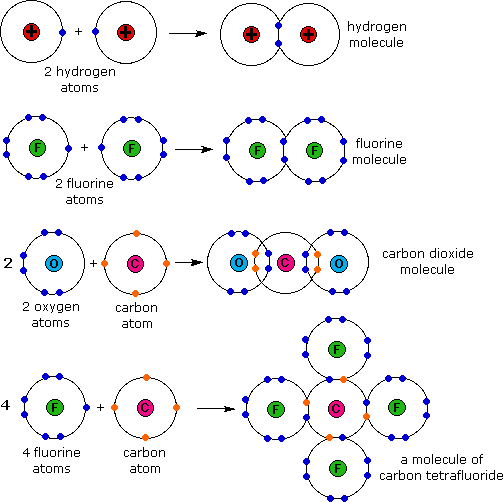
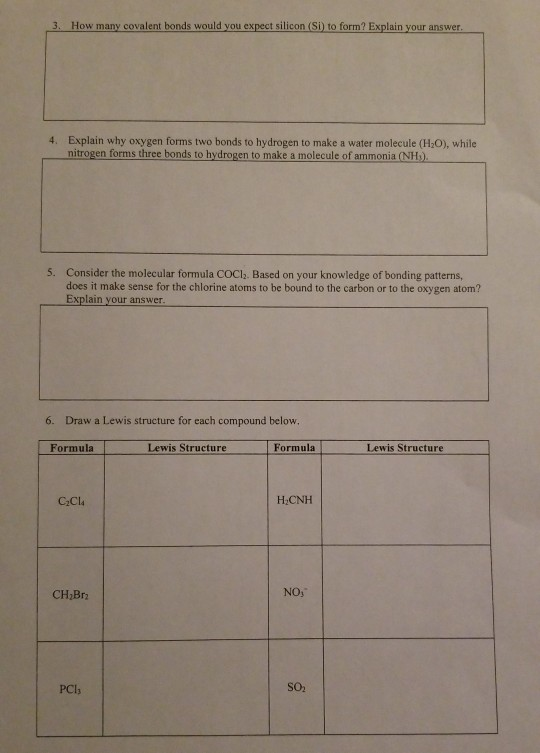
How Many Covalent Bonds Can Silicon Form
How Many Covalent Bonds Can Silicon Form - To obtain an octet, these. However, they all have slightly different properties and uses which we will. Web how many single covalent bonds must a silicon atom form to have a complete octet in its valence shell? Web giant covalent structures all consist of covalent bonds and are extremely strong. Web if life was based on an element which has 3 or 5 valence electron the variety in our life get reduced because carbon can form 4 single covalent bond where as nitrogen ( valence. Web how many covalent bonds can si form? Web the main point here is that a silicon atom has four electrons which it can share in covalent bonds with its neighbors. These simplified diagrams do not do justice to the nature of. Web it helps provide insight into the silicon silicon bond, but does not describe it as how silicon would bond with itself, but rather why it is ineffective. It takes two electrons to.
To obtain an octet, these. That means it can easily share all four of its valence electrons to form covalent. As a group 14 element, each silicon atom has four valence electrons. Prelude to covalent bonding and simple molecular compounds 4.3: Si is a nonmetal in group 4a, and. Web each silicon atom form si−si single bonds with four other atoms. Study guides chemistry 19 cards to name a monatomic anion change the. Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Silicon (si) is tetravalent in nature just like carbon (c). In a silicon crystal, how many covalent bonds does a single atom.
Web each silicon atom form si−si single bonds with four other atoms. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web simple molecules, which contain a fixed number of atoms. Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. To obtain an octet, these. Web 1.in silicon crystal the outer most class has 4 electrons.these 4 electrons are ready to pair to ge. Silicon (si) is tetravalent in nature just like carbon (c). The major (antisymmetric) component involves the radial. However, they all have slightly different properties and uses which we will. Web jul 30, 2020 4.1:
Covalent Bonds Biology for NonMajors I
Web each silicon atom form si−si single bonds with four other atoms. Web it helps provide insight into the silicon silicon bond, but does not describe it as how silicon would bond with itself, but rather why it is ineffective. Si is a nonmetal in group 4a, and. Web simple molecules, which contain a fixed number of atoms. Silicon (si).
How Many Single Bonds Can Carbon Form fredhughesdesign
There is a quick way to work out how many covalent bonds an element. That means it can easily share all four of its valence electrons to form covalent. These simplified diagrams do not do justice to the nature of. Web the main point here is that a silicon atom has four electrons which it can share in covalent bonds.
Solved How many covalent bonds can each element in the
Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web simple molecules, which contain a fixed number of atoms. Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Giant covalent substances, which contain many atoms joined by covalent bonds; Silicon (si) is tetravalent in.
Semiconductor Basics Tutorial Electrical Academia
Web how many single covalent bonds must a silicon atom form to have a complete octet in its valence shell? Si is a nonmetal in group 4a, and. Web small molecules, which contain a fixed number of atoms. Web jul 30, 2020 4.1: Web how many covalent bonds can si form?
Covalent Bond Biology Dictionary
That means it can easily share all four of its valence electrons to form covalent. The major (antisymmetric) component involves the radial. Web 1.in silicon crystal the outer most class has 4 electrons.these 4 electrons are ready to pair to ge. Giant covalent substances, which contain many atoms joined by covalent bonds; Web it helps provide insight into the silicon.
ASSTUDYPEACH Covalent Bonds Sharing Is Caring!
Web covalent bonding generally happens between nonmetals. It takes two electrons to. Silicon (si) is tetravalent in nature just like carbon (c). Si is a nonmetal in group 4a, and. Prelude to covalent bonding and simple molecular compounds 4.3:
Covalent Bonding (Biology) — Definition & Role Expii
The element silicon would be expected to form 4 covalent bond (s) in order to obey the octet rule. Web giant covalent structures all consist of covalent bonds and are extremely strong. These simplified diagrams do not do justice to the nature of. Web it helps provide insight into the silicon silicon bond, but does not describe it as how.
Solved 3. How many covalent bonds would you expect silicon
As a group 14 element, each silicon atom has four valence electrons. The element silicon would be expected to form 4 covalent bond (s) in order to obey the octet rule. Web how many single covalent bonds must a silicon atom form to have a complete octet in its valence shell? You'll get a detailed solution from a. To obtain.
__TOP__ How Many Covalent Bonds Can Chlorine Form
It takes two electrons to. The element silicon would be expected to form 4 covalent bond (s) in order to obey the octet rule. Giant covalent substances, which contain many atoms joined by covalent bonds; Web how many covalent bonds can si form? Web covalent bonding generally happens between nonmetals.
Is SiO2 Ionic or Covalent? Techiescientist
Web the main point here is that a silicon atom has four electrons which it can share in covalent bonds with its neighbors. However, they all have slightly different properties and uses which we will. In a silicon crystal, how many covalent bonds does a single atom. Covalent bonding is the type of bond that holds together the atoms within.
It Takes Two Electrons To.
Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Si is a nonmetal in group 4a, and. However, they all have slightly different properties and uses which we will. Web jul 30, 2020 4.1:
Web The Main Point Here Is That A Silicon Atom Has Four Electrons Which It Can Share In Covalent Bonds With Its Neighbors.
Web each silicon atom form si−si single bonds with four other atoms. Web it helps provide insight into the silicon silicon bond, but does not describe it as how silicon would bond with itself, but rather why it is ineffective. These simplified diagrams do not do justice to the nature of. To obtain an octet, these.
Web Simple Molecules, Which Contain A Fixed Number Of Atoms.
This problem has been solved! Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Web small molecules, which contain a fixed number of atoms. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4.
Web If Life Was Based On An Element Which Has 3 Or 5 Valence Electron The Variety In Our Life Get Reduced Because Carbon Can Form 4 Single Covalent Bond Where As Nitrogen ( Valence.
Web how many single covalent bonds must a silicon atom form to have a complete octet in its valence shell? There is a quick way to work out how many covalent bonds an element. Prelude to covalent bonding and simple molecular compounds 4.3: In a silicon crystal, how many covalent bonds does a single atom.