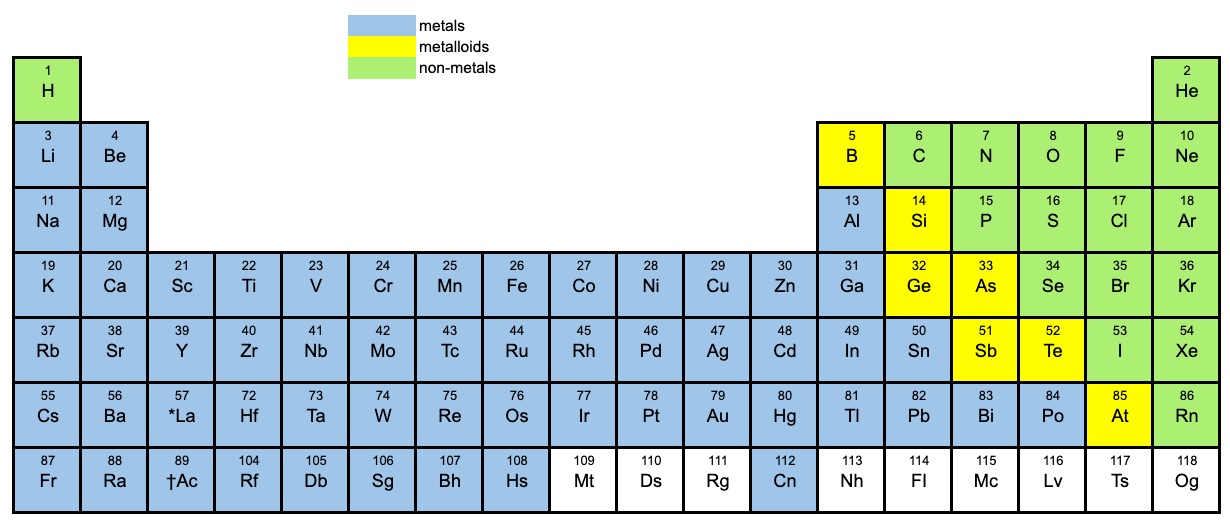
Do Metalloids Form Ionic Bonds
Do Metalloids Form Ionic Bonds - Web not sure who told you that, but metallic bonds can be just as strong as any ionic or covalent bonds. Web metalloids can form ionic bonds if they bond with metals. The molecule possessing ionic bond has. Ionic or interstitial compounds formed oxides: They can form covalent bonds by sharing electrons or ionic bonds by either losing or gaining electrons. Web transition metal ions do not follow an obvious pattern, 2 + is common, and 1 + and 3 + are also observed; Web my chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Many ionic compounds are referred to as salts as they can also be formed by the neutralization reaction of an. Web what kind of bonds do metalloids form? Web simply, metals lose electrons and can form only ionic bonds.
Metalloids are a covalent bond what is chemical bond, ionic bond, covalent bond? Ionic bond the bond formed by either losing or gaining an electron is called the ionic bond. Web my chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. Chemical bond a chemical bond is a lasting attraction between atoms, ions or. But sometimes, they form ionic compounds with other elements. Ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that generates two oppositely charged. Many ionic compounds are referred to as salts as they can also be formed by the neutralization reaction of an. What type of bond is between metals? Ionic bonds in general, covalent bonds form between nonmetals, ionic bonds. They can form covalent bonds by sharing electrons or ionic bonds by either losing or gaining electrons.
Web what kind of bonds do metalloids form? If they bond with non metals, they will have covalent bonds. Web metallic bonds are seen in pure metals and alloys and some metalloids. In reality all three of these bonds exist on a spectrum. The molecule possessing ionic bond has. Sio₂, silicon dioxide, is a covalent compound. It also depends on electronegativity of elements. Web metalloids can form covalent and ionic bonds. But sometimes, they form ionic compounds with other elements. Metalloids are a covalent bond what is chemical bond, ionic bond, covalent bond?
Ionic Bond Definition Easy Hard Science
In reality all three of these bonds exist on a spectrum. Web metalloids can form ionic bonds if they bond with metals. Web transition metal ions do not follow an obvious pattern, 2 + is common, and 1 + and 3 + are also observed; If they bond with non metals, they will have covalent bonds. Simply, metals lose electrons.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Ionic bonds in general, covalent bonds form between nonmetals, ionic bonds. Web not sure who told you that, but metallic bonds can be just as strong as any ionic or covalent bonds. If they bond with non metals, they will have covalent bonds. Web simply, metals lose electrons and can form only ionic bonds. It also depends on electronegativity of.
Ionic bonding Wikipedia
Web not sure who told you that, but metallic bonds can be just as strong as any ionic or covalent bonds. Web transition metal ions do not follow an obvious pattern, 2 + is common, and 1 + and 3 + are also observed; It also depends on electronegativity of elements. Ionic bond the bond formed by either losing or.
Ionic Bond Definition, Types, Properties & Examples
Web transition metal ions do not follow an obvious pattern, 2 + is common, and 1 + and 3 + are also observed; Web what kind of bonds do metalloids form? Ionic bond the bond formed by either losing or gaining an electron is called the ionic bond. But in another textbook i read that lithium,. Web metalloids can form.
Ionic Properties
In reality all three of these bonds exist on a spectrum. Simply, metals lose electrons and can form only ionic bonds. Web metalloids can form ionic bonds if they bond with metals. Web metallic bonds are seen in pure metals and alloys and some metalloids. But sometimes, they form ionic compounds with other elements.
Ionic Bond Definition, Types, Properties & Examples
Web metals and nonmetals are involved in ionic bonding, but it specifically refers to two elements that are oppositely charged being bonded to eachother. Web metallic bonds are seen in pure metals and alloys and some metalloids. Web simply, metals lose electrons and can form only ionic bonds. What type of bond is between metals? The molecule possessing ionic bond.
1. Naming compounds High School/Honors/AP® Chemistry
Are compounds with metalloids ionic or covalent? Metalloids are a covalent bond what is chemical bond, ionic bond, covalent bond? The molecule possessing ionic bond has. In reality all three of these bonds exist on a spectrum. Chemical bond a chemical bond is a lasting attraction between atoms, ions or.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
It also depends on electronegativity of elements. Chemical bond a chemical bond is a lasting attraction between atoms, ions or. Are compounds with metalloids ionic or covalent? Ionic bond the bond formed by either losing or gaining an electron is called the ionic bond. What type of bond is between metals?
Ionic Bond Definition, Types, Properties & Examples
Sio₂, silicon dioxide, is a covalent compound. What type of bond is between metals? Are compounds with metalloids ionic or covalent? But in another textbook i read that lithium,. Ionic bonds in general, covalent bonds form between nonmetals, ionic bonds.
Is SiO2 Ionic or Covalent? Techiescientist
Simply, metals lose electrons and can form only ionic bonds. Web what kind of bonds do metalloids form? Web my chemistry textbook says that metals form ionic or cordinate bonds whereas non metals form covalent bonds. But in another textbook i read that lithium,. But sometimes, they form ionic compounds with other elements.
Ionic Bonding Is The Complete Transfer Of Valence Electron (S) Between Atoms And Is A Type Of Chemical Bond That Generates Two Oppositely Charged.
Chemical bond a chemical bond is a lasting attraction between atoms, ions or. In reality all three of these bonds exist on a spectrum. Web metalloids can form ionic bonds if they bond with metals. It also depends on electronegativity of elements.
Sio₂, Silicon Dioxide, Is A Covalent Compound.
But sometimes, they form ionic compounds with other elements. Web metallic bonds are seen in pure metals and alloys and some metalloids. Many ionic compounds are referred to as salts as they can also be formed by the neutralization reaction of an. Metalloids are a covalent bond what is chemical bond, ionic bond, covalent bond?
Web What Kind Of Bonds Do Metalloids Form?
Ionic or interstitial compounds formed oxides: Ionic bonds in general, covalent bonds form between nonmetals, ionic bonds. If they bond with non metals, they will have covalent bonds. Web not sure who told you that, but metallic bonds can be just as strong as any ionic or covalent bonds.
The Molecule Possessing Ionic Bond Has.
But in another textbook i read that lithium,. Web transition metal ions do not follow an obvious pattern, 2 + is common, and 1 + and 3 + are also observed; Web metalloids can form covalent and ionic bonds. They can form covalent bonds by sharing electrons or ionic bonds by either losing or gaining electrons.