Chapter 7 Review Chemistry
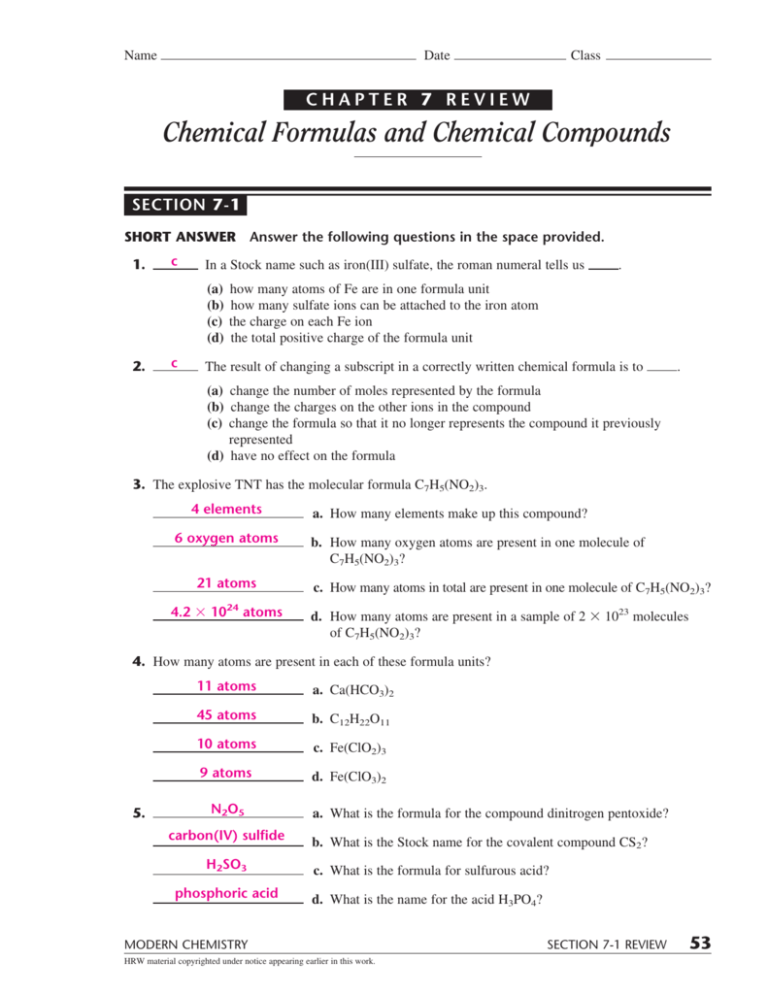
Chapter 7 Review Chemistry - Limiting reactant click the card to flip 👆 a. Web chemistry chapter 7 review hno click the card to flip 👆 hyponitrous acid click the card to flip 👆 1 / 19 flashcards learn test match created by clarbour teacher terms in this set (19) hno hyponitrous acid h3po3. Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry. Web 1) naming compounds 2) writing formulas 3) balancing chemical equations 4) studying chemical reactions what 3 types of information are used to find an empirical formula from percentage composition. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. What is the major conceptual difference between valence bond theory of main group compounds with. A chemical equation represents a. 4.2 ́ 1024 atoms a. Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: (b) how many sulfate ions can be attached to the iron atom.
More about atoms moles and molar mass isotopes unit 3: What is the major conceptual difference between valence bond theory of main group compounds with. What should a good bonding theory be able to do? The protons in the nucleus do not change during normal chemical reactions. Web create your own quiz. (b) how many sulfate ions can be attached to the iron atom. Click the card to flip 👆 a chemical formula includes the symbols of the elements in the compound. Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: The block has a constant acceleration and is pulled by means of a horizontal spring that. A chemical equation represents a.
A chemical equation represents a. Web chapter 7 chemistry review flashcards learn test match chemical formula click the card to flip 👆 shows the kinds and numbers of atoms in the smallest representative unit of a substance click the card to flip 👆 1 / 19 flashcards learn test match created by jackballard vocab chemistry. Web chemistry chapter 7 review 5.0 (1 review) b. Positive charges form when electrons are lost. (b) how many sulfate ions can be attached to the iron atom. Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry. The protons in the nucleus do not change during normal chemical reactions. What should a good bonding theory be able to do? Mar 21, 2022 | attempts: Web created by hlaney04 terms in this set (18) chemical formula indicates the relative number of atoms of each kind in a chemical compound monatomic ion ions formed from a single atom (many main group elements).
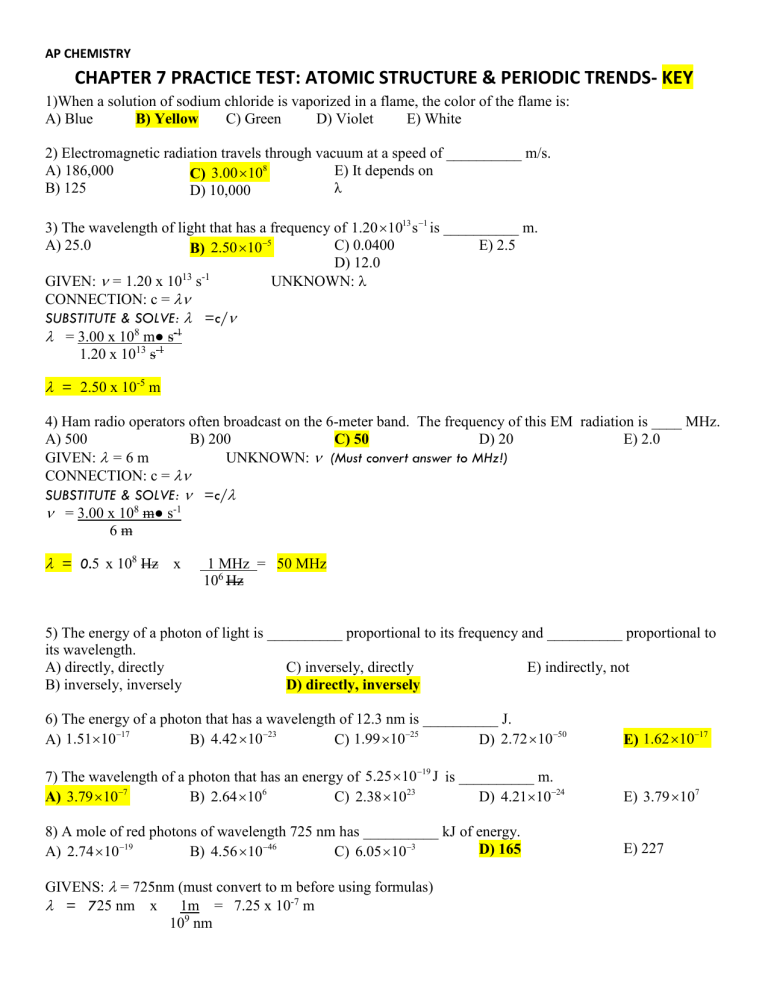
AP CHEMISTRY CHAPTER 7 PRACTICE TEST ATOMIC
A chemical equation represents a. Mar 21, 2022 | attempts: Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry. More about atoms moles and molar mass isotopes unit 3: Web quick summary of chapter 7:
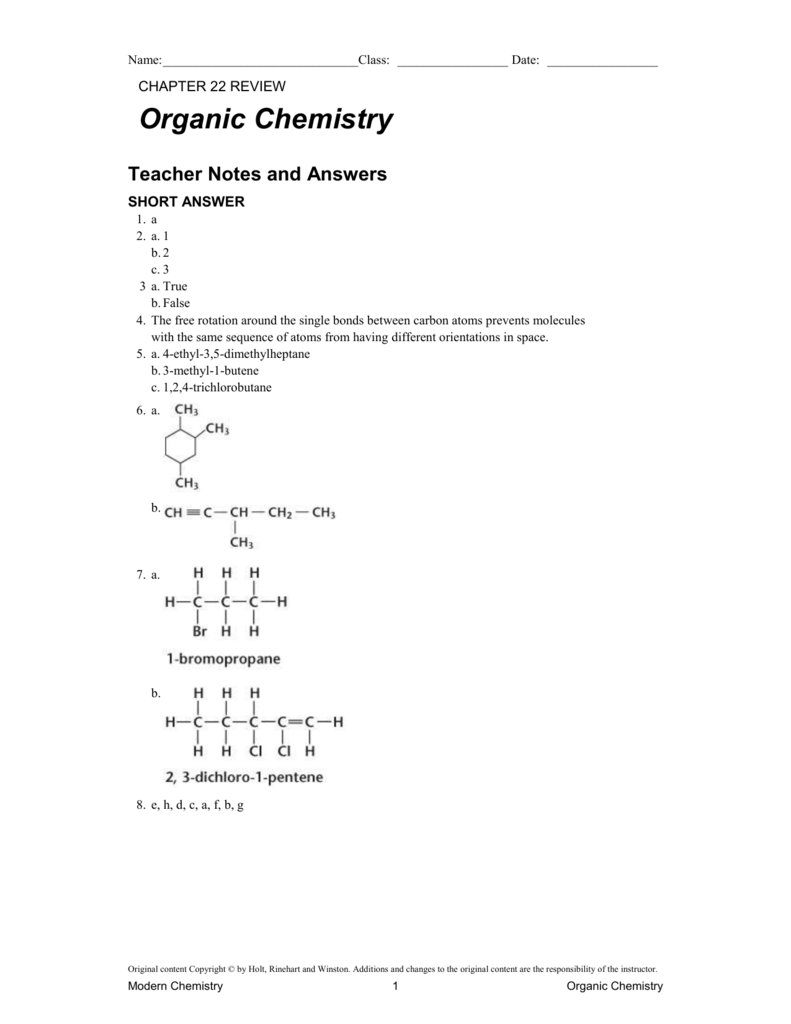
chapter 22 review
Click the card to flip 👆 a chemical formula includes the symbols of the elements in the compound. The protons in the nucleus do not change during normal chemical reactions. Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry. Web create your own quiz. Web created by hlaney04 terms in this set (18) chemical formula.
Chemistry, 4th edition Tests Answer Key Christian Liberty
Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry. Web chemistry chapter 7 review 5.0 (1 review) b. Web created by hlaney04 terms in this set (18) chemical formula indicates the relative number of atoms of each kind in a chemical compound monatomic ion ions formed from a single atom (many main group elements). The.
Modern Chemistry Chapter 7 Review Answers JackiChuhdary
The protons in the nucleus do not change during normal chemical reactions. Only the outer electrons move. Web chemistry chapter 7 review 5.0 (1 review) b. Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: (c) the charge on each fe ion.
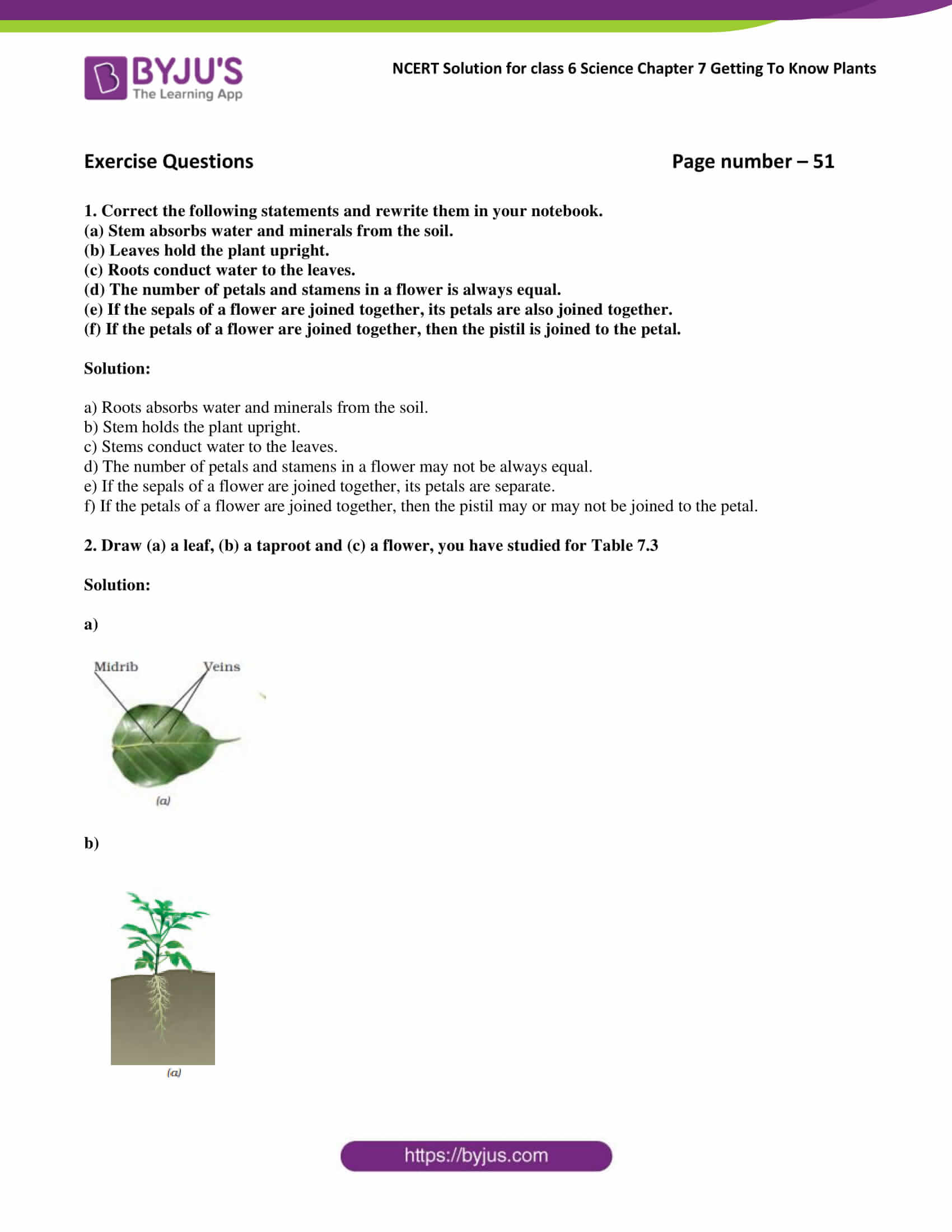
Selina Concise Chemistry Class 8 ICSE Solutions Chapter 1 Matter
More about atoms moles and molar mass isotopes unit 3: Web chapter 7 chemistry review flashcards learn test match chemical formula click the card to flip 👆 shows the kinds and numbers of atoms in the smallest representative unit of a substance click the card to flip 👆 1 / 19 flashcards learn test match created by jackballard vocab chemistry..
5 Best Images of Biochemistry Study Guides And Worksheets Protein
(d) the total positive charge of the formula. Positive charges form when electrons are lost. Web create your own quiz. Web 1) naming compounds 2) writing formulas 3) balancing chemical equations 4) studying chemical reactions what 3 types of information are used to find an empirical formula from percentage composition. Click the card to flip 👆 a chemical formula includes.
PPT Chapter 7 Review PowerPoint Presentation, free download ID2860726
What should a good theory be able to do? The block has a constant acceleration and is pulled by means of a horizontal spring that. Click the card to flip 👆 a chemical formula includes the symbols of the elements in the compound. Web create your own quiz. Web chemistry chapter 7 review 5.0 (1 review) b.
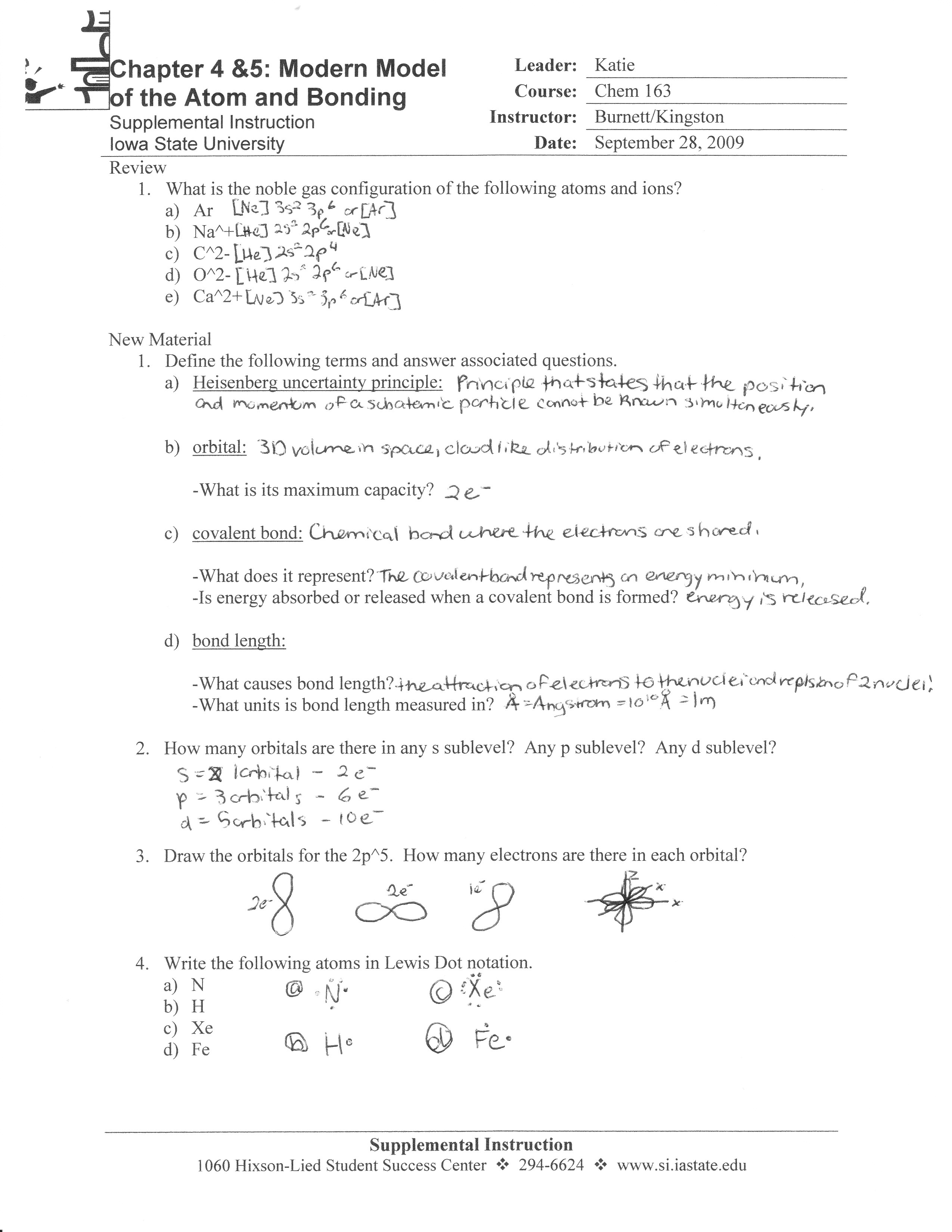
Chapter 7 Review
Web created by hlaney04 terms in this set (18) chemical formula indicates the relative number of atoms of each kind in a chemical compound monatomic ion ions formed from a single atom (many main group elements). Web chapter 7 chemistry review flashcards learn test match chemical formula click the card to flip 👆 shows the kinds and numbers of atoms.
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
More about atoms moles and molar mass isotopes unit 3: Positive charges form when electrons are lost. 18 questions | by muitran | updated: Energy is defined as the ability to do work. A chemical equation represents a.
Chapter 7 Test Review 4 YouTube
Atoms, compounds, and ions introduction to the atom ions and compounds names and formulas of ionic compounds unit 2: Write formulas for the following compounds: Web short answer answer the following questions in the space provided. Limiting reactant click the card to flip 👆 a. Web 1) naming compounds 2) writing formulas 3) balancing chemical equations 4) studying chemical reactions.
Web Chapter 7 Chemistry Review Flashcards Learn Test Match Chemical Formula Click The Card To Flip 👆 Shows The Kinds And Numbers Of Atoms In The Smallest Representative Unit Of A Substance Click The Card To Flip 👆 1 / 19 Flashcards Learn Test Match Created By Jackballard Vocab Chemistry.
Web 1) naming compounds 2) writing formulas 3) balancing chemical equations 4) studying chemical reactions what 3 types of information are used to find an empirical formula from percentage composition. (b) how many sulfate ions can be attached to the iron atom. Click the card to flip 👆 a chemical formula includes the symbols of the elements in the compound. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit.
Web Short Answer Answer The Following Questions In The Space Provided.
The block has a constant acceleration and is pulled by means of a horizontal spring that. (d) the total positive charge of the formula. Web quick summary of chapter 7: Thermochemistry is a branch of chemistry that studies the relationship between energy and chemistry.
Limiting Reactant Click The Card To Flip 👆 A.
(c) the charge on each fe ion. Mar 21, 2022 | attempts: Web chemistry chapter 7 review hno click the card to flip 👆 hyponitrous acid click the card to flip 👆 1 / 19 flashcards learn test match created by clarbour teacher terms in this set (19) hno hyponitrous acid h3po3. 4.2 ́ 1024 atoms a.
18 Questions | By Muitran | Updated:
Energy is defined as the ability to do work. Web created by hlaney04 terms in this set (18) chemical formula indicates the relative number of atoms of each kind in a chemical compound monatomic ion ions formed from a single atom (many main group elements). What should a good bonding theory be able to do? A chemical equation represents a.