Chapter 4 Review Arrangement Of Electrons In Atoms
Chapter 4 Review Arrangement Of Electrons In Atoms - Know aufbau principle, hund’s rule, & pauli. Web compare and contrast the bohr model and the quantum mechanical model of the atom. Web chemistry chapter 4 arrangement of electrons in atoms review. _____ compared with an electron for which. Web 0:00 / 9:09 chem in 15 minutes or less chapter 4: Web a) 1 b) 2 c) 3 d) 4, a spherical electron cloud surrounding an atomic nucleus would best represent: Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web learning objectives describe how electrons are grouped within atoms. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle.
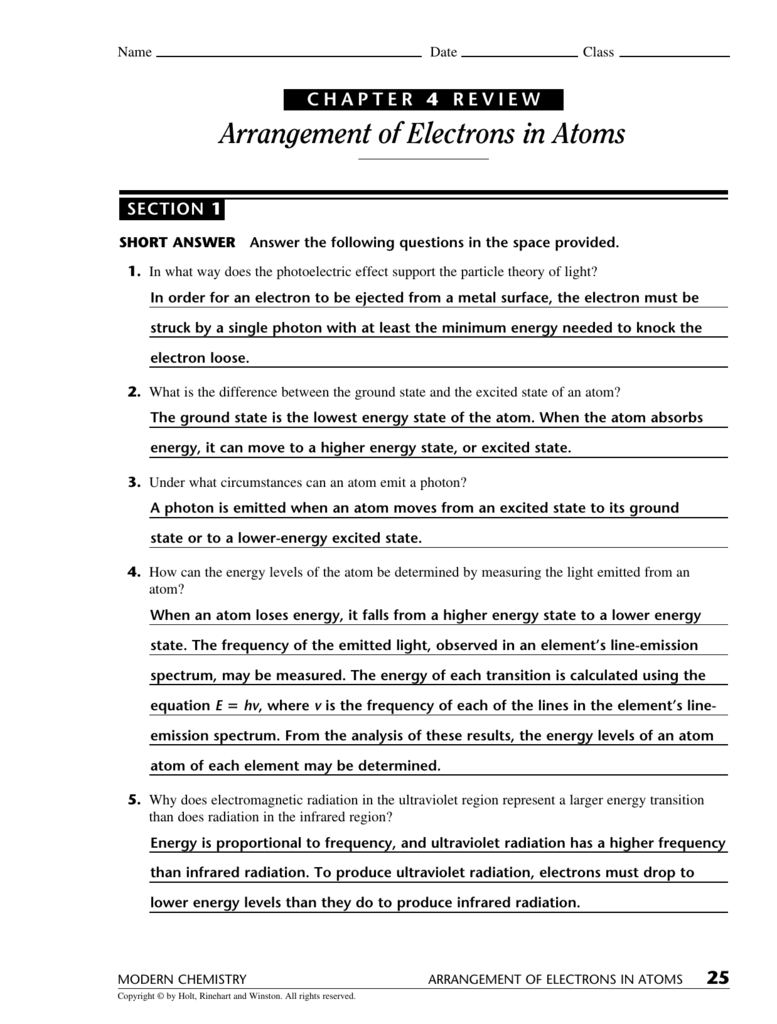
(a) 32 (c) 8 (b) 24 (d) 6 5. When the visible portion of the emitted light is passed. _____ how many electrons can an energy level of n = 2 hold? Bohr model says that electrons are in. Web chemistry chapter 4 arrangement of electrons in atoms review. Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4. The product of the frequency and the. _____ compared with an electron for which. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web chapter 4 test understand the differences in the energies of different orbital sublevels.
Although we have discussed the general arrangement of. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web 0:00 / 9:09 chem in 15 minutes or less chapter 4: The product of the frequency and the. Web learning objectives describe how electrons are grouped within atoms. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle. Web hydrogen atoms emit a pinkish glow, as is shown in this diagram. Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons. A) an s orbital b) a p orbital c) a. Web chapter 4 review arrangement of electrons in atoms section 2 short answer answer the following.
Arrangement of Electrons in Atoms
Web chapter 4 test understand the differences in the energies of different orbital sublevels. Web compare and contrast the bohr model and the quantum mechanical model of the atom. _____ how many electrons can an energy level of n = 2 hold? Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web.
PPT Chapter 5 Arrangement of electrons in atoms PowerPoint
Web learning objectives describe how electrons are grouped within atoms. The product of the frequency and the. (a) 32 (c) 8 (b) 24 (d) 6 5. Know aufbau principle, hund’s rule, & pauli. A) an s orbital b) a p orbital c) a.
5.2 Electron Arrangement in Atoms
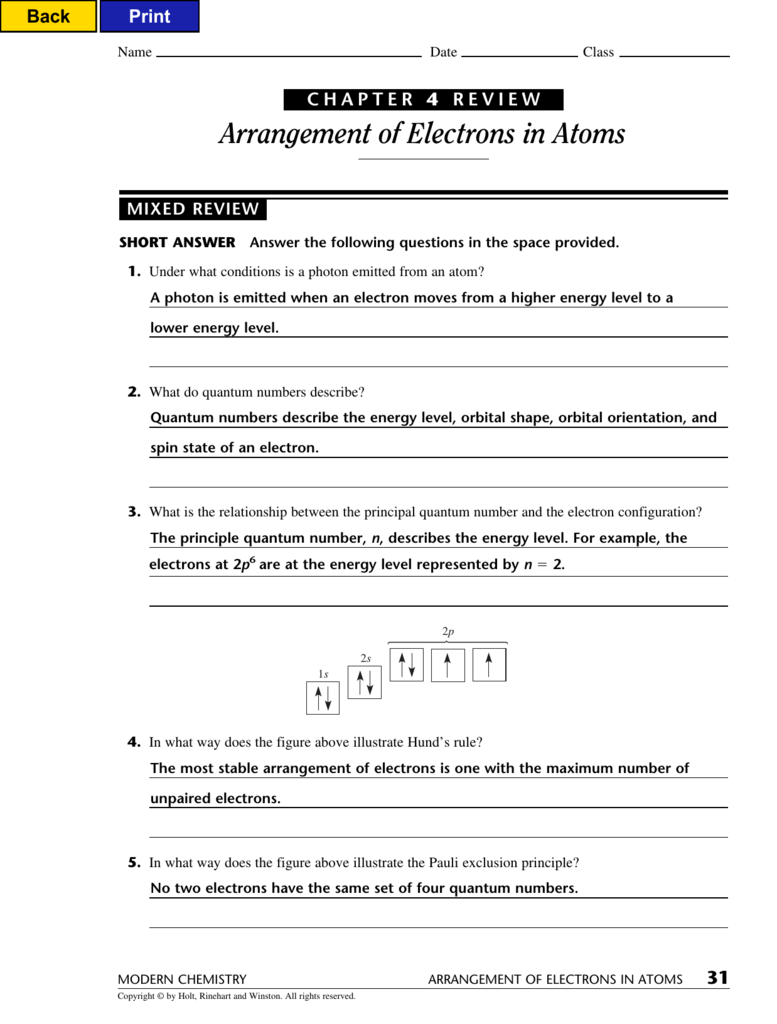
Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web chapter 4 review arrangement of electrons in atoms section 2 short answer answer the following. No two electrons can have the. Web a state in which an atom has a higher potential energy than it has in its ground state. Arrangement of.
Chapter 4 electrons in atoms
The product of the frequency and the. _____ compared with an electron for which. Web compare and contrast the bohr model and the quantum mechanical model of the atom. Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4. Although we have discussed the general arrangement of.
Ch. 4 Arrangement of Electrons in Atoms Crossword WordMint
Definition, sources & properties electromagnetic waves are both electric and magnetic, and can. When the visible portion of the emitted light is passed. No two electrons can have the. Although we have discussed the general arrangement of. Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons.
Arrangement of Electrons in Atoms
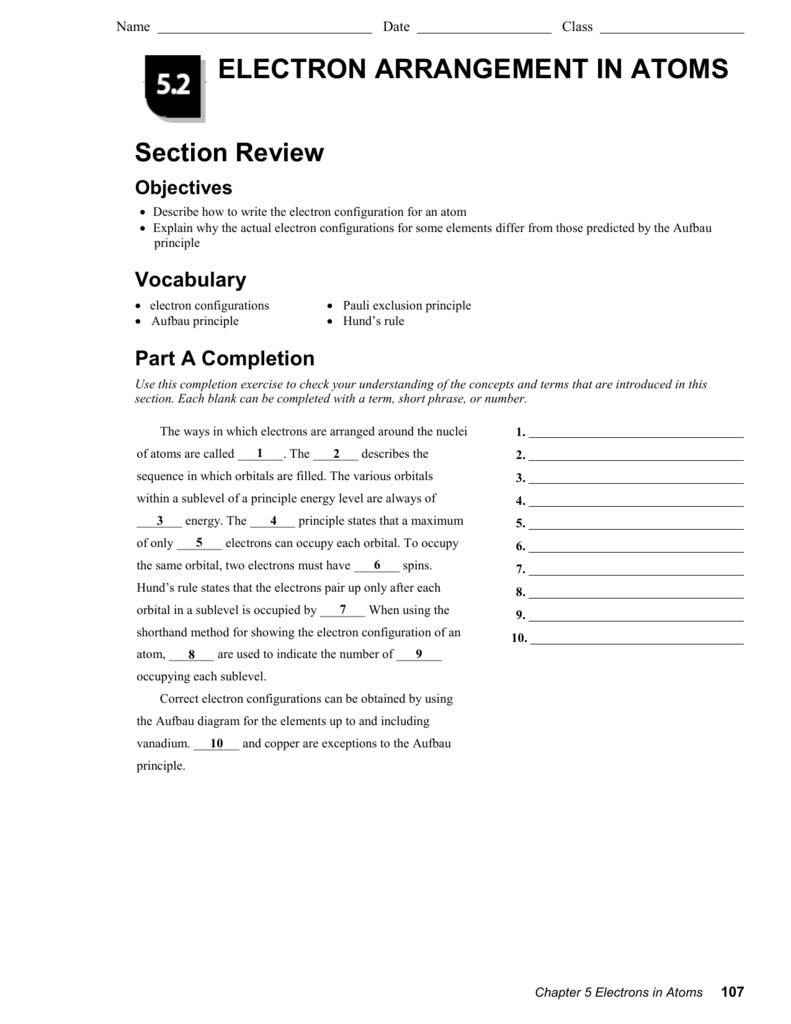
Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Know aufbau principle, hund’s rule, & pauli. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle. Web compare and contrast the bohr model and the quantum mechanical model of the atom. Web a) 1 b) 2 c) 3 d).
PPT Chapter 4 Arrangement of Electrons in Atoms PowerPoint
Web hydrogen atoms emit a pinkish glow, as is shown in this diagram. No two electrons can have the. Web chemistry chapter 4 arrangement of electrons in atoms review. Bohr model says that electrons are in. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle.
Chapter 4 electrons in atoms
Web a) 1 b) 2 c) 3 d) 4, a spherical electron cloud surrounding an atomic nucleus would best represent: Bohr model says that electrons are in. A) an s orbital b) a p orbital c) a. Web chapter 4 review arrangement of electrons in atoms section 2 short answer answer the following. Arrangement of electrons in atoms get a.
PPT Chemistry Chapter 4 Arrangement of Electrons in Atoms PowerPoint
_____ how many electrons can an energy level of n = 2 hold? Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Web hydrogen atoms emit a pinkish glow, as is shown in this diagram. _____ compared with an electron for which. (a) 32 (c) 8 (b) 24 (d) 6 5.
Chapter 4 Review Arrangement Of Electrons In Atoms Answer Key
Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle. Although we have discussed the general arrangement of. Web chemistry chapter 4 arrangement of electrons in atoms review. _____ compared with an electron for which. A) an s orbital b) a p orbital c) a.
Know Aufbau Principle, Hund’s Rule, & Pauli.
Web learning objectives describe how electrons are grouped within atoms. A) an s orbital b) a p orbital c) a. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web hydrogen atoms emit a pinkish glow, as is shown in this diagram.
Web A State In Which An Atom Has A Higher Potential Energy Than It Has In Its Ground State.
Bohr model says that electrons are in. _____ compared with an electron for which. Web 0:00 / 9:09 chem in 15 minutes or less chapter 4: Although we have discussed the general arrangement of.
Web Chemistry Arrangement Of Electrons In Atoms Chapter 4 Test Review Term 1 / 112 As An Electron Moves From N=1 To N=4.
No two electrons can have the. Web compare and contrast the bohr model and the quantum mechanical model of the atom. When the visible portion of the emitted light is passed. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle.
Web A) 1 B) 2 C) 3 D) 4, A Spherical Electron Cloud Surrounding An Atomic Nucleus Would Best Represent:
(a) 32 (c) 8 (b) 24 (d) 6 5. Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons. _____ how many electrons can an energy level of n = 2 hold?