Can Polar Molecules Form Hydrogen Bonds
Can Polar Molecules Form Hydrogen Bonds - When polar molecules are near each. Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. What is the difference between hydrogen bonds and polar bonds? No , they are different. Web hydrogen bonding is explained as the intermolecular forces between polar molecules. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. The most common species for x. This means the molecules will be soluble in a polar solvent such. Web because the hydrogen bond occurs between polar regions of a molecule, it is, like all polar attractions, relatively weak. Hence it makes a strong hydrogen bond.
No , they are different. Hydrogen bonding is a special type of dipole force between highly polarized molecules. When polar molecules are near each. Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. Web therefore, it makes the molecules polar. A simple example of hydrogen bonding can be seen. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. This means the molecules will be soluble in a polar solvent such. The most common species for x. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar.
Web answer (1 of 4): Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. Web because the hydrogen bond occurs between polar regions of a molecule, it is, like all polar attractions, relatively weak. Web a polar molecule is similar to a magnet, it has a positively charged side and a negatively charged side on the opposite side. What is the difference between hydrogen bonds and polar bonds? Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or. This means the molecules will be soluble in a polar solvent such. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. When polar molecules are near each.
PPT Why Study Chemistry? PowerPoint Presentation ID1433229
Web nov 19, 2015. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. Web answer (1 of 4): What is the difference between hydrogen bonds and polar bonds? Hydrogen bonding is a special type of dipole force between highly polarized molecules.
How Do Polar Molecules Form Hydrogen Bonds? Sciencing
The most common species for x. Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. A simple example of hydrogen bonding can be seen. Web the water molecules at an interface of apolar material are strongly oriented.
PPT Properties of Water PowerPoint Presentation, free download ID
Web a polar molecule is similar to a magnet, it has a positively charged side and a negatively charged side on the opposite side. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. This means the molecules will be soluble in a polar solvent such. The most common species for x. Web thus far.
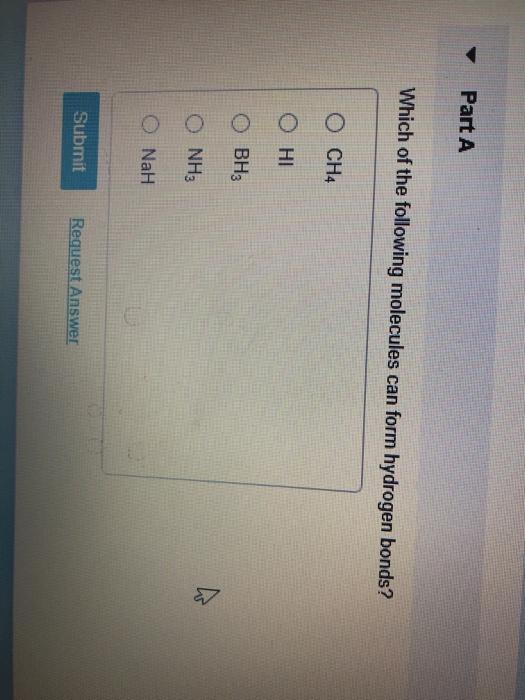
Solved Part A Which of the following molecules can form
Hydrogen bonds are intermolecular forces rather than forces within a molecule. No , they are different. When polar molecules are near each. No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f.
In hydrogen bonds, do both molecules have to be polar? Quora
Web answer (1 of 4): Web nov 19, 2015. The most common species for x. Hence it makes a strong hydrogen bond. The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f.
Learn for free about math, art, computer programming, economics
The most common species for x. Web nov 19, 2015. When polar molecules are near each. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. Web hydrogen bond strengths range from 4 kj to 50 kj per mole.
11 Types of scientific changes with examples
Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. Web therefore, it makes the molecules polar. Web nov 19, 2015. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. No, but first.
Covalent Bonds Biology for NonMajors I
When polar molecules are near each. Web nov 19, 2015. Web therefore, it makes the molecules polar. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. No , they are different.
Water Review
Web nov 19, 2015. Web hydrogen bonding is explained as the intermolecular forces between polar molecules. The most common species for x. Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or. Hence it makes a strong hydrogen.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or. Web answer (1 of 4): This means the molecules will be soluble.
Hydrogen Bonding Is A Special Type Of Dipole Force Between Highly Polarized Molecules.
What is the difference between hydrogen bonds and polar bonds? Web because the hydrogen bond occurs between polar regions of a molecule, it is, like all polar attractions, relatively weak. Web thus far we have considered only interactions between polar molecules, but other factors must be considered to explain why many nonpolar molecules, such as. Web the hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment x−h in which x is more electronegative than h, and an atom or.
Web Hydrogen Bond Strengths Range From 4 Kj To 50 Kj Per Mole Of Hydrogen Bonds.
Web hydrogen bonding is explained as the intermolecular forces between polar molecules. Hydrogen bonds are intermolecular forces rather than forces within a molecule. Web the water molecules at an interface of apolar material are strongly oriented so as to form as many hydrogen bonds as possible to other water molecules, as none can be. No , they are different.
The Most Common Species For X.
Web the presence of hydrogen bonding between molecules of a substance indicates that the molecules are polar. Web therefore, it makes the molecules polar. Hence it makes a strong hydrogen bond. This means the molecules will be soluble in a polar solvent such.
Web A Polar Molecule Is Similar To A Magnet, It Has A Positively Charged Side And A Negatively Charged Side On The Opposite Side.
No, but first i should clarify what i mean by hydrogen bonds, and polar bonds. Hydrogen bond arises when hydrogen is attached to high electronegative elements like f o n. When polar molecules are near each. The hydrogen bond in polar molecules occurs only in compounds that have hydrogen bonded to n, o, or f.