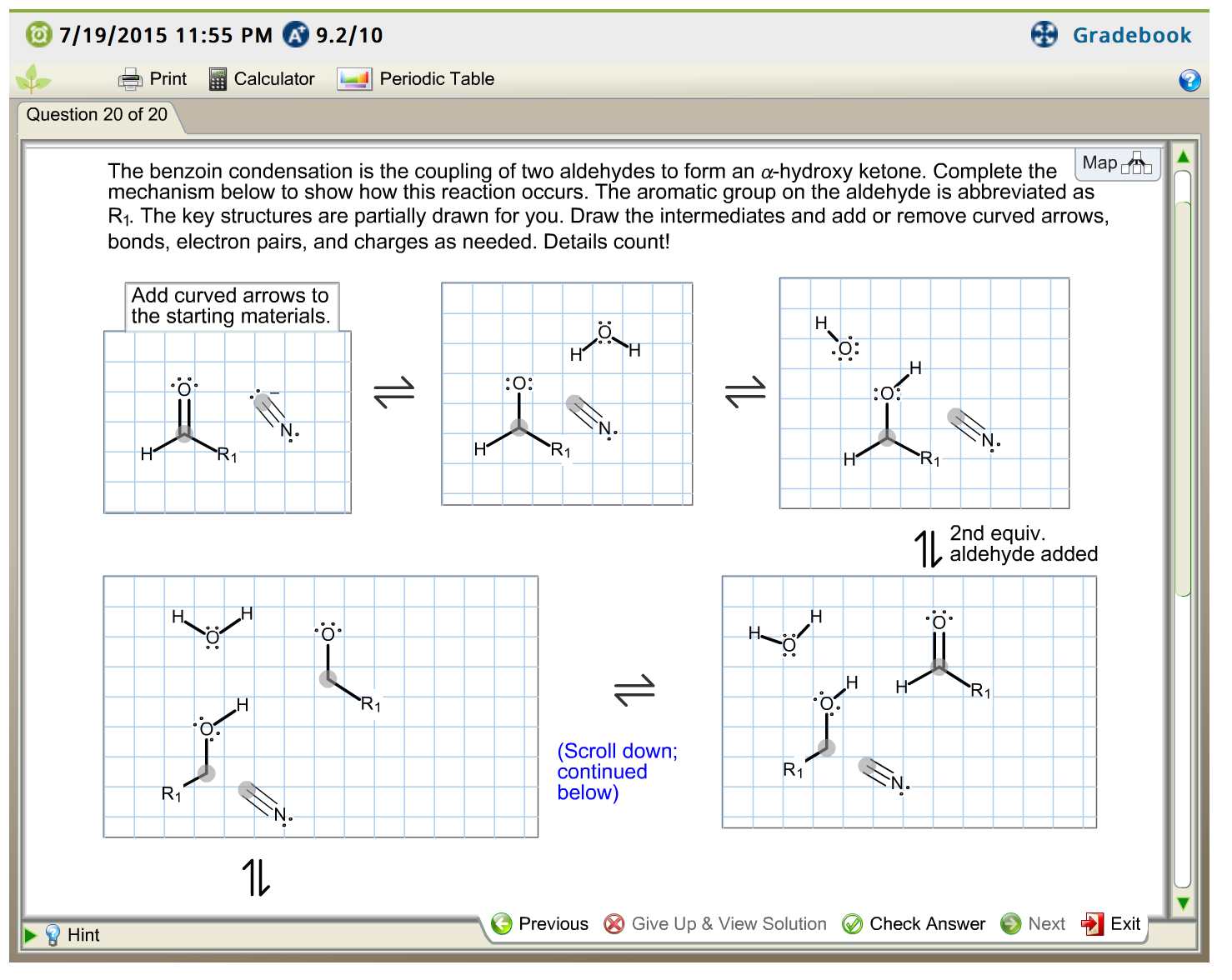
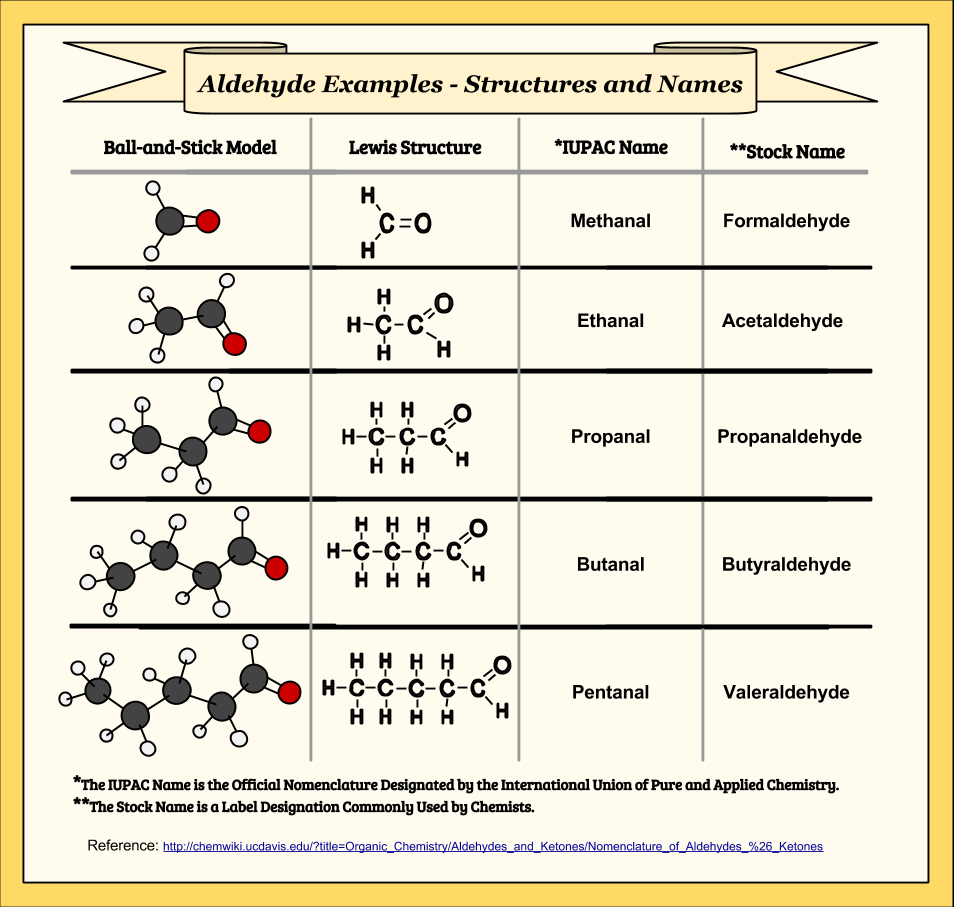
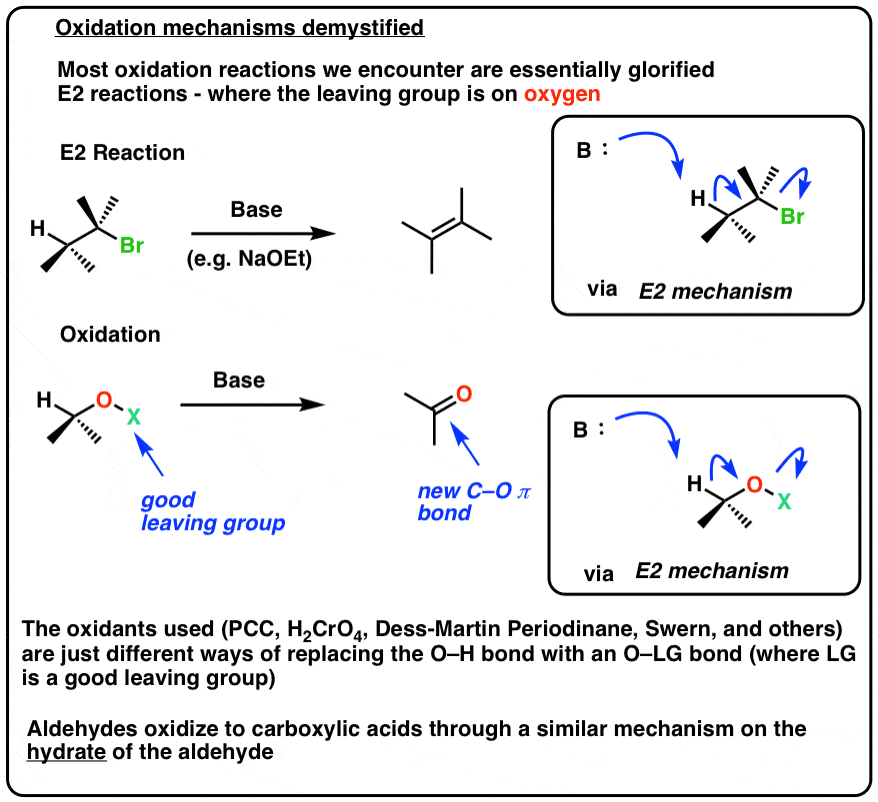
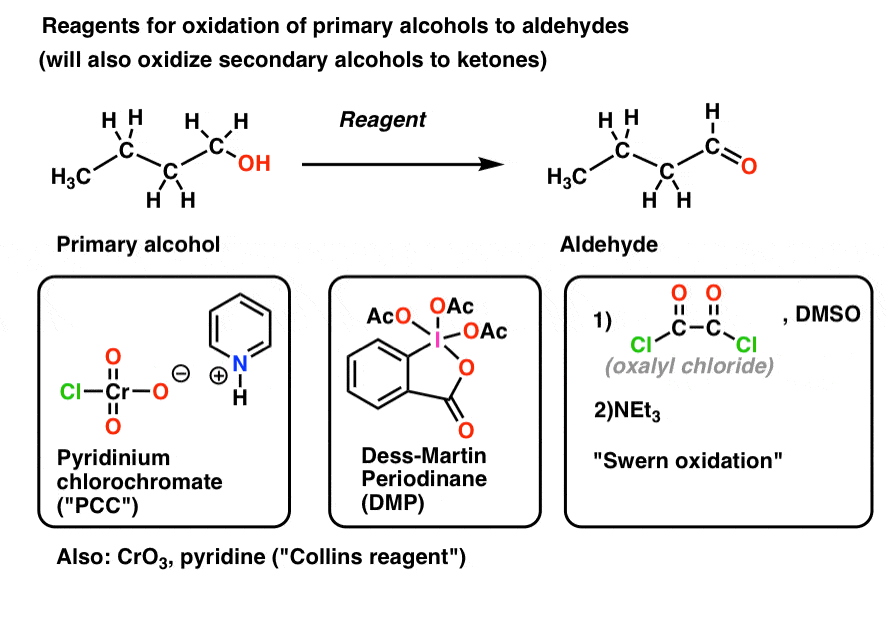
Aldehydes May Oxidize To Form
Aldehydes May Oxidize To Form - Web the oxidation of an alcohol to form an aldehyde or ketone is very important in synthesis. In this process, the hydroxy hydrogen of the alcohol is replaced by a leaving group (x in. They use a strong oxidant like potassium permanganate (kmno4) Hence, option b is correct. This will happen if the oxidation happens under acidic or alkaline conditions. Web ¥carbonyl groups in aldehydes and ketones may be oxidized to form compounds at the next òoxidation level ó, that of carboxylic acids. Aldehydes are further oxidized to carboxylic acids. Web aldehydes, rcho, can be oxidised to carboxylic acids, rco2h. Web depending on the conditions of the oxidation, aldehydes will form carboxylic acids. Web the product of the oxidation of an aldehyde, results in a carboxylic acid.
Oxidation of alcohols to aldehydes is partial oxidation; Web the oxidation of aldehydes by o2 appears to be a chain mechanism involving free radicals and yielding a rather reactive peracid, which then reacts with the. Web ¥carbonyl groups in aldehydes and ketones may be oxidized to form compounds at the next òoxidation level ó, that of carboxylic acids. In other words, aldehydes are better at reducing than ketones due to the presence of the hydrogen. Web there are some reagents which can selectively oxidize a primary alcohol and stop at an aldehyde without complete oxidation to the acid. Web answer 1 aldehydes are a class of organic compound which can be oxidized to form corresponding carboxylic acid or organic acid. Web oxidation of 1 o alcohols to form aldehydes (section 17.7) pcc pyridinium chlorochromate ( pcc) is a milder version of chromic acid. This will happen if the oxidation happens under acidic or alkaline conditions. These functional groups are useful for further reactions; Hence, option b is correct.
Oxidation of alcohols to aldehydes is partial oxidation; Web oxidation of alcohols to aldehydes and ketones. This will happen if the oxidation happens under acidic or alkaline conditions. Web there are some reagents which can selectively oxidize a primary alcohol and stop at an aldehyde without complete oxidation to the acid. These functional groups are useful for further reactions; Web aldehydes reduce the diamminesilver(i) ion to metallic silver. Alcohols may be oxidized to give ketones, aldehydes, and carboxylic acids. Hence, option b is correct. In this case, excess dichromate will further oxidize the aldehyde to a. Web ¥carbonyl groups in aldehydes and ketones may be oxidized to form compounds at the next òoxidation level ó, that of carboxylic acids.
Solved The benzoin condensation is the coupling of two
Web oxidation can be achieved by heating the alcohol with an acidified solution of potassium dichromate. Web aldehydes undergo oxidation more quickly than ketones. Hence, option b is correct. O c h o c o h oxidation ¥alcohols. Aldehydes are further oxidized to carboxylic acids.
Solved Be sure to answer all parts. Hydroxy aldehydes A and
In this process, the hydroxy hydrogen of the alcohol is replaced by a leaving group (x in. Web currently, most investigations on aldehyde oxidations focus on aerobic oxidation, i.e., using molecular oxygen (o 2) to oxidize aldehydes into the corresponding carboxylic. Web the oxidation of aldehydes by o2 appears to be a chain mechanism involving free radicals and yielding a.
Representative examples of oxidation of aldehydes. Download
Aldehydes are further oxidized to carboxylic acids. Web aldehydes undergo oxidation more quickly than ketones. Oxidation of alcohols to aldehydes is partial oxidation; Web currently, most investigations on aldehyde oxidations focus on aerobic oxidation, i.e., using molecular oxygen (o 2) to oxidize aldehydes into the corresponding carboxylic. In this process, the hydroxy hydrogen of the alcohol is replaced by a.
PPT Aldehydes, Ketones, and carboxylic acids PowerPoint Presentation
Web there are some reagents which can selectively oxidize a primary alcohol and stop at an aldehyde without complete oxidation to the acid. Web aldehydes have a proton attached to the carbonyl carbon which can be abstracted, allowing them to be easily oxidized to form carboxylic acids. In this process, the hydroxy hydrogen of the alcohol is replaced by a.
Organic Chemistry, Form, Part 14 Aldehydes Structure and
Because the solution is alkaline, the aldehyde itself is oxidized to a salt of the corresponding carboxylic acid. Ketones are not oxidised under these conditions as they lack the critical h for the elimination to occur (see. Primary alcohols can only be oxidized to form aldehydes or carboxylic acids.for instance, using chromic acid (to get a carboxylic acid), or using.
PDF Télécharger aldehydes may oxidize to form Gratuit PDF
Web aldehydes undergo oxidation more quickly than ketones. Web answer 1 aldehydes are a class of organic compound which can be oxidized to form corresponding carboxylic acid or organic acid. Web the product of the oxidation of an aldehyde, results in a carboxylic acid. Oxidation of alcohols to aldehydes is partial oxidation; Web depending on the conditions of the oxidation,.
Alcohol Oxidation Mechanisms and Practice Problems Chemistry Steps
Web aldehydes undergo oxidation more quickly than ketones. Web the product of the oxidation of an aldehyde, results in a carboxylic acid. Web there are some reagents which can selectively oxidize a primary alcohol and stop at an aldehyde without complete oxidation to the acid. O c h o c o h oxidation ¥alcohols. Web oxidation can be achieved by.
Alcohol Oxidation "Strong" & "Weak" Oxidants Master Organic Chemistry
Web depending on the conditions of the oxidation, aldehydes will form carboxylic acids. Ketones are not oxidised under these conditions as they lack the critical h for the elimination to occur (see. O c h o c o h oxidation ¥alcohols. In this process, the hydroxy hydrogen of the alcohol is replaced by a leaving group (x in. Web currently,.
Pathways leading to formation of oxidation and nitric oxide (NO
Web the product of the oxidation of an aldehyde, results in a carboxylic acid. In this case, excess dichromate will further oxidize the aldehyde to a. Web the oxidation of aldehydes by o2 appears to be a chain mechanism involving free radicals and yielding a rather reactive peracid, which then reacts with the. Web aldehydes, rcho, can be oxidised to.
Tollens Reagent Silver Mirror Test for Aldehydes
Web the oxidation of aldehydes by o2 appears to be a chain mechanism involving free radicals and yielding a rather reactive peracid, which then reacts with the. Web answer 1 aldehydes are a class of organic compound which can be oxidized to form corresponding carboxylic acid or organic acid. In other words, aldehydes are better at reducing than ketones due.
In This Case, Excess Dichromate Will Further Oxidize The Aldehyde To A.
Web aldehydes, rcho, can be oxidised to carboxylic acids, rco2h. Web oxidation can be achieved by heating the alcohol with an acidified solution of potassium dichromate. O c h o c o h oxidation ¥alcohols. Alcohols may be oxidized to give ketones, aldehydes, and carboxylic acids.
Web Answer 1 Aldehydes Are A Class Of Organic Compound Which Can Be Oxidized To Form Corresponding Carboxylic Acid Or Organic Acid.
In this process, the hydroxy hydrogen of the alcohol is replaced by a leaving group (x in. Web oxidation of 1 o alcohols to form aldehydes (section 17.7) pcc pyridinium chlorochromate ( pcc) is a milder version of chromic acid. Web the oxidation of an alcohol to form an aldehyde or ketone is very important in synthesis. Web the product of the oxidation of an aldehyde, results in a carboxylic acid.
Hence, Option B Is Correct.
Web aldehydes have a proton attached to the carbonyl carbon which can be abstracted, allowing them to be easily oxidized to form carboxylic acids. Web the oxidation of aldehydes by o2 appears to be a chain mechanism involving free radicals and yielding a rather reactive peracid, which then reacts with the. Web aldehydes undergo oxidation more quickly than ketones. Web ¥carbonyl groups in aldehydes and ketones may be oxidized to form compounds at the next òoxidation level ó, that of carboxylic acids.
Web Oxidation Of Alcohols To Aldehydes And Ketones.
Oxidation of alcohols to aldehydes is partial oxidation; These functional groups are useful for further reactions; Web currently, most investigations on aldehyde oxidations focus on aerobic oxidation, i.e., using molecular oxygen (o 2) to oxidize aldehydes into the corresponding carboxylic. In other words, aldehydes are better at reducing than ketones due to the presence of the hydrogen.